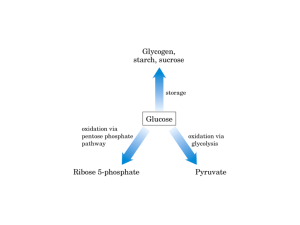
3. Biotechnological Importance of MO - Copy
advertisement

Development of industrial fermentation processes • • • • • Money making Competition Economically feasible on large scale basis Recovery of product ready for open market Competitive advantage Criteria for being important in choice of organism 1. Nutritional characteristics of the organism when grown on a cheap medium 2. Optimum temp of the organism 3. Reaction of the organism with the equipment and suitability for the type of process 4. Stability of the organism and its amenability for genetic manipulation 5. Productivity of the organism i.e. ability to convert substrate into product per unit time 6. Ease of product recovery from the culture What are the R&D approaches for finding of a MO of economic value, and large scale fermentation process? Micro-organism Stock culture collections Source Screening Primary screening Secondary screening Environment (soil) Primary screening • Highly selective procedures for detection and isolation of MO of interest • Few steps will allow elimination of valueless MO • Eg. Crowded plate technique for Ab screening, serial dilution, acid base indicator dyes, CaCO3, sole source carbon or nitrogen, enrichment tech • Does not give too much information on detail ability of the microorganisms • May yield only a few organisms and few of them may have commercial value Common techniques I. 1. 2. 3. 4. 5. 6. Direct wipe or sponge of the soil Soil dilution (10-1 to 10-10) Gradient plate method (streak, pour) Aerosol dilution Flotation Centrifugation II. Enrichment, screening for metabolites or microbial products III. Unusual environments Secondary screening • Sorting of MO that have real commercial value for industrial processes and discarding those which lack potential • Conducted on agar plates (not sensitive), small flasks or small fermentors (more sensitive) containing liquid media or combination of these approaches. • Liquid culture provide better info on nutritional, physical and production responses. • Can be qualitative or quantitative Preservation of Industrially important MO • Viable and Free from contamination • Stored in such a way so as to eliminate genetic change and retain viability • Viable by repeated sub-culture (avoid mutations by keeping stocks and strain degeneration and contaminations) Preservation of Industrially important MO 1. Storage at reduced temperature a. Agar slopes at 50C or in -200C freezer: viable for 6 months b. Liquid nitrogen (-1960C): problems of refilling, advantages 2. Storage at dehydrated form a. Dried cultures b. Lyophillization Quality control of preserved stock: batch system, single colony, typical pattern, large number, purity, viability and productivity If sample fails entire batch is destroyed MICROBIAL METABOLIC PRODUCTS OR METABOLITES • Wide range of products having commercial value Algae SCP Bacteria acetic acid bactracin gramicidin endotoxin glutamic acid vitamin B12 Actinomycetes antibiotics (tetracycline, streptomycin, neomycin, rifamycin, gentamycin) Fungi citric acid, amylase, cellulase, SCP, lipase, pencillin, ethanol, wine, steroids, gibberllin TYPES OF LOW MOLECULAR WEIGHT COMPOUNDS BY MO SUBSTRATE Primary metabolites Secondary metabolites Antibiotics Essential metabolites Amino acids Nucleosides vitamins Ethanol, acetone, lactic acid, butanol Steroids Amino acids Alkaloids Gibberlins Pigments Metabolic end products Bioconversions Ascorbic acid Primary metabolism Secondary metabolism Idiophase Trophophase Concentration Cell Mass Limiting nutrient Secondary metabolite Time PRIMARY METABOLITES Formed in trophophase (log phase) Balanced growth of MO Occurs when all nutrients are provided in the medium Its is essential for survival and existence of the organism and reproduction Cells have optimum concentration of all macromolecules (proteins, DNA, RNA etc.) Exponential growth PRIMARY METABOLITES 1. Primary essential metabolites: • • • • Produced in adequate amount to sustain cell growth Vitamins, amino acids, nucleosides These are not overproduced, wasteful Overproduction can be genetically manipulated 2. Primary essential end products: • • • Normal end products of fermentation process of primary metabolism Not have a significant function in MO but have industrial applications Ethanol, acetone, lactic acid, CO2 LIMITATIONS: growth rate slows down due to limited supply of any other nutrient. Metabolism does not stop but product formation stops. OVERPRODUCTION OF PRIMARY METABOLITES Manipulation of feedback inhibition • Auxotrophic mutants having a block in steps of a biosynthetic pathway for the formation of primary metabolite (intermediate not final end prod). End product formation is blocked and no feedback inhibition • Mutant MO with defective metabolite production Unbranched pathway intermediate A ---- > B ----> C -----> D ------> E Starting substrate Final end prod Blocked reaction Required metabolite SECONDARY METABOLITES idiophase • Characterized by secondary metabolism and secondary metabolites (idolites) • Produced in abundance, industrially important Characteristics: 1. 2. 3. 4. Specifically produced Non essential for growth Influenced by environmental factors Some produce a group of compds eg a strain of Streptomyces produced 35 anthracyclines 5. Biosynthetic pathways are not established 6. Regulation of formation is more complex Functions: 1. May or may not contribute for existence or survival of the MO OVERPRODUCTION OF SECONDARY METABOLITES More complex Several genes are involved eg may be 300 to 2000 genes Regulatory systems are more complex Some regulatory mechanisms 1. Induction: eg tryptophan for ergot production etc 2. End product regulation: some metabolite inhibit their own biosysnthesis 3. Catabolite regulation: key enzyme inactivated, inhibited or repressed eg. Glucose can inhibit several antibiotics ammonia as inhibitor for antibiotic prod. 4. Phosphate regulation: Pi for growth and multiplication in pro and eukaryotes. Increase in pi conc can increase secondary metabolites but excess harmful 5. Autoregulation: self regulation mechanism for production like hormones BIOCONVERSIONS OR BIOTRANSFORMATIONS Used for chemical transformation of unusual substrates for desired prods Conversion of ethanol to acetic acid, sorbitol to sorbose, synthesis of steroid hormones and certain amino acids Structurally related compounds in one or few enzymatic reactions Can use resting cells, spores or even killed cells. Mixed cultures can also be used, use of immobilized cells at low cost ? BIOCONVERSIONS OR BIOTRANSFORMATIONS (BTs) When and why is biotransformation done? when production of a particular compound is difficult or costly by chemical methods BTs are preferred over chemical reactions due to substrate specificity, stereospecificity, mixed reaction conditions (pH, temp, pressure) Environmental pollution is negligible Easy to apply recombinant DNA technology Easy to scale up the processes sue to limited number of reactions TYPES OF HIGH MOLECULAR WEIGHT COMPOUNDS BY MO Polysaccharides, proteins (enzymes) Pharmaceutical products Enzymes naturally occurring biocatalysts; accelerate metabolic reactions Production of primary and secondary metabolites are not possible without enzymes Enzymes during fermentation are EXTRACELLULAR (amylase, cellulase, lipase, b-galactosidase, esterase, protease, chitinase, xylanase, glucose isomerase) and some are INTRACELLULAR (invertase, asparginase) Extremozymes Immobilized enzymes Microbial Biomass Microbes can themselves be products or main source of biomass Microbial biomass is exploited as microbial protein or single cell protein (SCP) METABOLIC PATHWAYS IN MICRO-ORGANISMS 1. PROVIDES PRECURSORS FOR THE CELL COMPONENTS 2. ENERGY FOR ENERGY REQUIRING PROCESSES Unique feature of heterotrophic MO Secrete extracellular enzymes 1. Catabolism 2. Amphibolism (Intermediate metabolism requiring central metabolic pathways) 3. Anabolism 4. Function of enzymes: substrate specificity, catalysis 5. Coenzymes and prosthetic group 6. Methods of ATP generation: SLP, OP (respy), OP (photosyn) 7. Uptake of substrates (diffusion, FD, AT, Gp Trans, siderophores 8. Degradation of carbon and energy sources (sugar breakdown) METABOLIC PATHWAYS IN MICRO-ORGANISMS Carbon and energy source breakdown Sugars to Pyruvate The ways in which microorganisms degrade sugars to pyruvate and similar intermediates are introduced by focusing on only three routes: (1) Glycolysis (Embden Meyerhof Pathway) (2) The pentose phosphate pathway, (3) The Entner-Doudoroff pathway (1) Glycolysis: glucose to pyruvate Hexokinase 6-carbon phase phosphofructokinase Fructose biphosphate aldolase oxidation phase energy harvest phase Glucose KDPG Pathway Or Entner Dourdoff Pathway Glucose 6 Phosphate Pyruvic acid Acetyl CoA Pentose phosphate pathway Centre of Intermediate metabolism Precursor for Numerous Biosynthetic pathways (2) Entner-Doudoroff pathway or KDPG pathway Glucose ----> 2 pyruvic acid + ATP +NAD(P)H2 + NADH2 Glucose 6 phosphogluconate dehydrase Glucose-6-P ATP 6 Phosphogluconolactone NADPH 6-phosphogluconate instead of Fructose 6-P 2-keto-3-deoxyphosphogluconate aldolase 2-keto-3-deoxy-6-phosphogluconate (KDPG) Embden-Meyerhof pathway Pyruvate glyceraldehyde-3-P Pyruvate Only in prokaryotes, many gram negative bacteria some G+ve Operates when glycolytic enzymes like phosphofructokinase-1 are lacking 1 net ATP is produced 1 NADPH and 1 NADH is also produced 2ATP 1 NADH (3) Pentose Phosphate pathway (PPP) or HMP Heterofermenter lactobacilli Bacteria which lack aldolase for conversion to triose phosphate PPP takes place Oxidative catabolism of glucose Dehydrogenation hydrolysis Reducing equivalents * Glucose 6 phosphate * * * * glycolysis To Microbial fermentation pathways CO2 LAF NADH acetaldehyde EthanolAF MxAF BuDF BuAF PropAF BuAcetoneF BuAcidF METABOLIC PATHWAYS IN MICRO-ORGANISMS Precursors for biosynthesis 3C 2C 4C NADH 6C 4C 6C 4C 5C 4C GTP CO2 4C CO2 NADH CO2 NADH LMW constituents Glycolysis Pentose phosphate pathway Entner Douordoff pathway Krebs cycle Purines Pyrimidines Lipids Phospholipids Amino acids Macromolecular constituents Glycolysis Pentose phosphate pathway Entner Douordoff pathway Krebs cycle DNA, RNA Proteins Peptidoglycans Polysaccharides (glycogen, starch, PHB) Glucose ATP PPP G-6-P AA syn Histidine Tryptophan Ribose 5P Tyrosine Erythrose 4P Phenylalanine pyruvate Gly Serine Cys Ala Leu Oxaloacetate Val Lys a keto glutarate Glu --- Gln Pro AROMATIC AA GLUTAMATE FAMILY Ori -- citruline -- Arg DAP Asp SERINE FAMILY PYRUVATE FAMILY ASPARTATE FAMILY Asn Homoserine - Met Thr ----- Ile