Chapter 6
advertisement

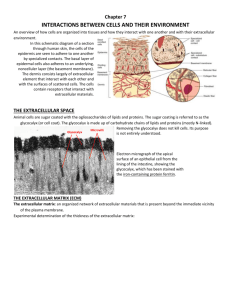
Fundamentals of Cell Biology Chapter 6: The Extracellular Matrix and Cell Junctions iClicker Time If cell biologists use the term “GTP cap” when discussing microtubules, why don’t they use the term “ATP cap” when discussing actin filaments? A. ATP-bound actin monomers do not polymerize. B. All actin monomers in an actin filament are bound to ATP. C. Actin filaments do not undergo dynamic instability in cells. D. Actin filament severing proteins cut the ATP cap off so quickly it is usually not detectable. E. Actin biologists don’t consider depolymerization of an actin filament a catastrophe. Chapter Summary: The Big Picture (1) • Chapter foci: – Examine representative molecules that are commonly found in the space between cells, the extracellular matrix, which are highly specialized to perform distinct functions in the extracellular spaces and in cell–extracellular matrix junctions – Examine the molecules that form direct links between cells, cell–cell junctions, with an introduction to several different kinds of cell–cell junctions Chapter Summary: The Big Picture (2) • Section topics: – The extracellular matrix is a complex network of molecules that fills the spaces between cells in a multicellular organism – Cells adhere to one another via specialized proteins and junctional complexes The extracellular matrix (EM) is a complex network of molecules that fills the spaces between cells in a multicellular organism • Key Concepts (1): – The extracellular matrix is a dense network of molecules that lies between cells in a multicellular organism and is made by the cells within the network. – The principal function of collagen is to provide structural support to tissues. – The principal function of fibronectin is to connect cells to matrices that contain fibrillar collagen. – The principal function of elastin is to impart elasticity to tissues. The extracellular matrix (EM) is a complex network of molecules that fills the spaces between cells in a multicellular organism • Key Concepts (2): – The principal function of laminins is to provide an adhesive substrate for cells and to resist tensile forces in tissues. – Proteoglycans consist of a central protein “core” to which long, linear chains of disaccharides, called glycosaminoglycans (GAGs), are attached. The extracellular matrix (EM) is a complex network of molecules that fills the spaces between cells in a multicellular organism • Key Concepts (3): – The basal lamina is a thin sheet of EM found at the basal surface of epithelial sheets and at neuromuscular junctions and is composed of at least two distinct layers. – Cells express receptors for EM molecules. Virtually all animal cells express integrins, which are the most abundant and widely expressed class of EM protein receptors. Glycoproteins form filamentous networks between cells • Collagen provides structural support to tissues Basic unit: coiled coil 4 classes:Type I-IV Figure 06.01: Collagen subunits are assembled into triple-helical coiled coils. Figure 06.02: Collagens are organized into four major classes, which vary according to their molecular formula, polymerized form, and tissue distribution. Structure of collagen fibers • 3 polypeptide subunits wrapped in parallel to form a 300-nm-long coiled coil • characteristic repeat sequence consisting of glycine-X-Y Figure 06.03: Schematic diagram of collagen triple-helical coiled coil (top), organization of coiled coils within a fibril (middle), and fibrils in a collagen fiber (bottom). Collagen assembly Figure 06.04: Posttranslational modification and assembly of procollagen subunits. Fibronectins connect cells to collagenous matrices Figure 06.05: Two fibronectin polypeptides are covalently linked via disulfide bonds near the carboxyl terminus. • fibronectin repeats • classified into three groups - Type I, II, III • mechanism of fiber assembly unclear but believed that fibronectin dimers first bind to cell surface receptors called integrins Figure 06.06: The fibronectin dimer is secreted in a folded conformation that is stabilized by interactions between fibronectin repeats I1-5, III2-3 and III12-14. Elastic fibers impart flexibility to tissues • Elastin is organized into elastic fibers, which consist of a core region enriched in elastin proteins surrounded by a tough coating called a microfiber (or microfibrillar) sheath Figure 06.08: Schematic representation of relaxed and stretched elastic fibers. Current model of elastin fibrilogenesis Figure 06.09: Seven steps of elastin fiber assembly. Laminins provide an adhesive substrate for cells • 3 polypeptide subunits wrapped together to form a triple helical coiled coil • each subunit extends “arms” out from the coil giving rise to a cross-shaped structure Figure 06.10: The three chains of the laminin molecules are wrapped into a central core. Proteoglycans provide hydration to tissues • provide tensile strength ensuring EM is hydrated gel • GAGs • >40 different core proteins identified • each contains modular structural domains that can bind to components of EM Figure 06.12: Summary of proteoglycan structures. Figure 06.15: Proteoglycans such as aggrecan complex with collagen II fibers in cartilage. Hyaluronan is a GAG enriched in connective tissues • binds to proteoglycan aggrecan • creates large, hydrated spaces in the EM of cartilage Figure 06.15: Proteoglycans such as aggrecan complex with collagen II fibers in cartilage. The basal lamina is a specialized EM • lies immediately adjacent to, and in contact with, many cell types • contains proteins (collagen IV and nidogen) found only in this structure • adopts distinct, sheet-like arrangement • “basement membrane” Figure 06.16: Hemisdesmosomes connect to the basement membrane, which consists of the basal lamina and a network of collagen fibers. Figure 06.17: The basement membrane. Caption A: The basement membrane appears as a thin layer of protein immediately under epithelial cells. Most integrins are receptors for EM proteins • bind to EM proteins and membrane proteins expressed on surface of other cells • principal surface proteins for holding tissues together • complex structure • classified into 3 subfamilies based on β subunits Figure 06.18: Model of integrin structure. Figure 06.19: Integrins are organized into subgroups that share β subunits. Specialized integrin clusters play distinct roles in cells • clusters classified into 5 types • composition of cluster varies depending on type(s) of integrins in cluster, type of EM bound by integrins, degree of tensile strain imposed on cluster, location of cluster in cell, and type of cell in which cluster forms Figure 06.21: Five types of integrin clusters. Filopodia Figure 05.35: Different forms of actin in stationary and migrating cells. Integrins control a vast range of cellular functions Figure 06.23: Summary of integrin cluster components and the cellular activities they control. Hemidesmosomes • contain α6β4 integrin and link to the IF network • cell surface junction found at basal surface of plasma membrane of epithelial cells The Module • What you need to know: – What epidermolysis bullosa is, and what causes it – What the Central Dogma of Molecular Biology is, and how EB demonstrates it. – The difference between a hypothesis and a guess – The structure of a logical argument – How the data in the first research article (Module 1-2) contribute to our understanding of EB – How the data in Figure 1 of the second research article (Module 1-3) were generated, and what they reveal about the cause of EB. iClicker Time What structural property makes proteoglycans distinct from all other extracellular matrix molecules? A. They are polar and thus bind to water. B. They are not found in basement membranes. C. They contain no amino acids. D. They do not bind to any other cellular molecules. E. Their function is determined largely by the sugars they contain. Cells adhere to one another via specialized proteins and junctional complexes • Key Concepts (1): – Cell–cell junctions are specialized protein complexes that allow neighboring cells to adhere to and communicate with one another. – Tight junctions regulate transport of particles between epithelial cells and preserve epithelial cell polarity by serving as a “fence” that prevents diffusion of plasma membrane proteins between the apical and basal regions. – Adherens junctions are a family of related cell-surface domains that link neighboring cells together. Cells adhere to one another via specialized proteins and junctional complexes • Key Concepts (2): – The principal function of desmosomes is to provide structural integrity to sheets of epithelial cells by linking the IF networks of cells. – Hemidesmosomes are found on the basal surface of epithelial cells, where they link the EM to the IF network via transmembrane receptors. – Gap junctions are protein structures that facilitate direct transfer of small molecules between adjacent cells. They are found in most animal cells. Cells adhere to one another via specialized proteins and junctional complexes • Key Concepts (3): – Cadherins constitute a family of cell surface transmembrane receptor proteins that are organized into eight groups. The best-known group of cadherins, called classical cadherins, plays a role in establishing and maintaining cell–cell adhesion complexes such as the adherens junctions. Cells adhere to one another via specialized proteins and junctional complexes • Key Concepts (4): – Neural cell adhesion molecules (NCAMs) are expressed only in neural cells and function primarily as homotypic cell–cell adhesion and signaling receptors. – Selections are cell–cell adhesion receptors expressed exclusively on cells in the circulatory system. They arrest circulating immune cells in blood vessels so that they can crawl out into the surrounding tissue. Tight junctions form selectively permeable barriers between cells • junctional complex is made up of: – tight junction – adherens junction – desmosome Figure 06.25: The junctional complex is composed of at least three distinct cell-cell junctions. Tight junctions • 3 types of transmembrane proteins found in the tight junction: claudins, occludins, and the junctional adhesion molecule (JAM) • functions as a permeability barrier Figure 06.27: Tight junctions are held together by occludin, claudin, and junctional adhesion molecules. Figure 06.28: A model of fast and slow transport of solutes through tight junctions. Adherens junction • hold epithelial and endothelial cells together – resist stress • zonula adherens • adhesive junctions in synapses • intercalated disks between adjacent cardiac muscle cells • junctions between layers of myelin sheath Figure 06.30: The zonula adherens is part of the junctional complex. Figure 06.31: Each type of adherens junction functions to hold adjacent cells together tightly. Desmosome • thick accumulations of fibrils running across gap between two plasma membranes of epithelial cells • fibrils terminate in electrondense material on cytosolic side of plasma membrane • electron-dense patches are connected to filaments in cytosol of each cell Figure 06.33: Desmosome proteins are distributed in the plasma membrane and a distinctive double plaque arrangement at the cell surface. Gap junctions allow direct transfer of molecules between adjacent cells • cell-to-cell transport of ions and small molecules • connexons – 6 connexin subunits Figure 06.34: The principal structural unit of the gap junction is the connexon, which consists of six membranespanning connexin subunits. Calcium-dependent cadherins mediate adhesion between cells • 70 structurally-related transmembrane proteins • 2 properties: – 1) bind to calcium ions to fold properly (Ca, for calcium) – 2) adhere to other proteins (adherin) Figure 06.37: Cadherin cytoplasmic tails are linked to actin filaments via catenin proteins. Figure 06.38: As the neural tube is formed, the apical surface of the neural plate cells constricts, causing the neural plate to curve inward. Calcium-independent NCAMs mediate adhesion between neural cells Figure 06.39: NCAMs are produced as both membrane-bound and soluble proteins of different sizes. Figure 06.40: Strong and weak cellcell adhesion. Selectins control adhesion of circulating immune cells Figure 06.41: An illustration of the “rolling stop” function of selectins.