Lecture Slides 2
advertisement

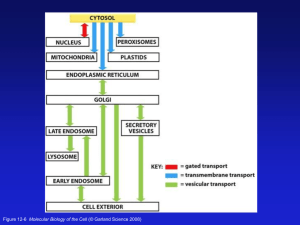
Chapter 12 • Intracellular Compartments and Protein Sorting Figure 12-1 Molecular Biology of the Cell (© Garland Science 2008) Protein Movement between Compartments * Most proteins are synthesized on cytoplasmic ribosomes and must be delivered to their ultimate compartment of residence. * Proteins contain sorting signals that direct their movement throughout the cell. * These sorting signals are recognized by specific receptors that mediate delivery to the appropriate organelle. * There are three major types of protein traffic between compartments: 1) Gated transport 2) Transmembrane translocation 3) Vesicular transport Figure 12-6 Molecular Biology of the Cell (© Garland Science 2008) Gated Transport (Nuclear Import/Export) Figure 12-10 Molecular Biology of the Cell (© Garland Science 2008) Transmembrane Translocation * Proteins are directly translocated across the membrane bilayer. * Translocation is performed by a membrane protein complex that forms a translocation pore. * Proteins pass through the membrane bilayer as unfolded chains. * Two major strategies are used to accomplish this feat: co-translational & post-translational import. Vesicular Transport Figure 12-7 Protein sorting signals Signal sequences direct protein delivery SS Destination Mitochondria Cytoplasm 1. Deletion of signal sequence (SS) Cytoplasm 2. Addition of a signal sequence Mitochondria SS Mitochondrial Protein Import * Mitochondria utilize the energy from electron transport and oxidative phosphorylation to synthesize the majority of the cell's ATP. * Most mitochondrial proteins are synthesized on cytoplasmic ribosomes and are post-translationally imported into this organelle. * Because of the double membrane surrounding this organelle, there are four targets for mitochondrial proteins: 1. Outer membrane 3. Inner membrane 2. Intermembrane space 4. Matrix space * Mitochondrial proteins usually contain an N-terminal targeting sequence that is capable of forming an amphipathic -helix; positively-charged residues are clustered on one side of the helix and uncharged residues are present on the other. * The mitochondrial outer membrane contains specific receptor proteins that bind to the mitochondrial targeting signal. The four compartments within mitochondria Figure 12-21 Molecular Biology of the Cell (© Garland Science 2008) * Signal sequence for mitochondrial protein import. * Note the amphipathic nature of the -helix. Figure 12-22 Protein translocators in mitochondrial membranes (Matrix/Inner Membrane) (Inner Membrane) Figure 12-23 Mitochondrial Protein Import (cont’d) * Translocation into the mitochondrial matrix requires both ATP hydrolysis and an electrochemical gradient across the inner mitochondrial membrane. * Translocation occurs at sites where the inner and outer membrane are in close apposition. These regions are known as contact sites. * Proteins are imported into the mitochondria in an unfolded state. * Maintenance in an unfolded state is mediated by hsp70 proteins that act as molecular chaperones. * Protein transport into the inner membrane or intermembrane space requires additional targeting signals. * Much of our current knowledge of mitochondrial protein import has come from in vitro studies with isolated mitochondria. Protein import into mitochondria Figure 12-25 Molecular Biology of the Cell (© Garland Science 2008) The role of energy in mitochondrial protein import Figure 12-26 Molecular Biology of the Cell (© Garland Science 2008) The hsp70 family of molecular chaperones Figure 6-86 Molecular Biology of the Cell (© Garland Science 2008) The role of energy in mitochondrial protein import Figure 12-26 Molecular Biology of the Cell (© Garland Science 2008) Figure 12-28 Molecular Biology of the Cell (© Garland Science 2008) Figure 12-28b Molecular Biology of the Cell (© Garland Science 2008) Figure 12-28 Molecular Biology of the Cell (© Garland Science 2008) Studying mitochondrial protein import in vitro * Isolated mitochondria are mixed with the radioactivelylabeled protein to be studied ? IMPORT ? IMPORT * Import may be detected by one of the following methods: 1) Density gradient centrifugation; if imported, proteins will fractionate with the organelle. 2) SDS-PAGE analysis to determine if the signal sequence was removed during the import reaction. 3) Protease protection assays; imported protein will be protected from the action of added proteases. * By adding or removing different components from the import reaction, one can determine the requirements for protein import. in vitro studies of mitochondrial protein import Figure 12-24 Molecular Biology of the Cell (© Garland Science 2008) Secretory Pathway Proteins enter into the secretory pathway at the ER where they are co-translationally inserted into the ER membrane. Proteins then travel to successive organelles via membrane-bound intermediates. Endoplasmic Reticulum Functions of the ER * The entry point for proteins that proceed through the secretory pathway. * Modification of proteins: a predominant modification is the glycosylation of specific asparagine residues (N-linked sugars). * Quality control: proteins must be properly folded before they are allowed to leave the ER. Proteins that fail to achieve a native state are degraded. * Sequestration of Ca++ from the cytoplasm. * Primary site of lipid biosynthesis. Figure 12-35 Molecular Biology of the Cell (© Garland Science 2008) Abundant smooth ER in steroid-hormonesecreting cell A 3-D reconstruction of ER in liver cell Figure 12-36c Molecular Biology of the Cell (© Garland Science 2008) Rough ER in pancreatic exocrine cell Free Membranebound Figure 12-41a Separating the Smooth & Rough ER Figure 12-37b Molecular Biology of the Cell (© Garland Science 2008) The Signal Hypothesis Figure 12-38 George Palade: 1974 Nobel Prize in Medicine Protein Import into the ER Step 1: Establishing a tight interaction with the ER membrane * A hydrophobic signal peptide, usually at the N-terminus of the protein, directs entry into the ER. * The signal peptide is recognized by the Signal Recognition Particle (SRP) as soon as it emerges from the ribosome. This interaction arrests translation. * The ER membrane contains an SRP receptor that mediates the initial association of the SRP-ribosome complex with the cytoplasmic face of the ER. * The ribosome subsequently associates with a translocation complex (the Sec61 complex) in the ER membrane and the SRP is released back into the cytosol. Table 12-3 Molecular Biology of the Cell (© Garland Science 2008) Signal-recognition particle (SRP) Figure 12-39a Two functions for the signal sequence: 1. Targets protein/ribosome complex to the ER membrane. 2. Serves as a start-transfer sequence that opens translocation pore. Figure 12-40 Molecular Biology of the Cell (© Garland Science 2008) Protein Import into the ER Step 2: Co-translational translocation of the polypeptide * Upon association with the ER, the ribosome resumes translation and co-translationally inserts the polypeptide chain into the ER lumen through a translocation pore. * The protein is passed through the membrane as a single, unfolded chain and folds into its native conformation within the ER lumen. This folding process requires protein chaperones. * The N-terminal signal peptide is removed by Signal Peptidase, a protease present in the lumen of the ER. * Integral membrane proteins contain "stop transfer" sequences that result in a block to the translocation process. Structure of the Sec61 translocation complex Figure 12-42 Molecular Biology of the Cell (© Garland Science 2008) A ribosome bound to the Sec61 protein translocator Figure 12-43 Molecular Biology of the Cell (© Garland Science 2008) Translocation of a soluble protein Figure 12-45 Molecular Biology of the Cell (© Garland Science 2008) A single-pass transmembrane protein Figure 12-46 Molecular Biology of the Cell (© Garland Science 2008) Integration of a singlepass membrane protein with an internal signal sequence NOTE: Orientation across membrane bilayer. Figure 12-47 Molecular Biology of the Cell (© Garland Science 2008) A double-pass transmembrane protein Figure 12-48 Molecular Biology of the Cell (© Garland Science 2008) Insertion of a multipass membrane protein into the ER Figure 12-49 Molecular Biology of the Cell (© Garland Science 2008) Genetic approaches for studying the mechanism of protein translocation Wild-type Engineered Cell Enzyme in cytosol: cell lives without histidine Enzyme targeted to ER: cell dies without histidine Mutant Engineered Cell Not all enzyme targeted to ER: cell lives without histidine Panel 12-1 * Most proteins in the secretory pathway are glycosylated; modified by the addition of sugar residues. * A precursor oligosaccharide unit is added to particular asparagine residues (N-linked carbohydrate). Figure 12-50 Molecular Biology of the Cell (© Garland Science 2008) Protein glycosylation in the rough ER Figure 12-51 Synthesis of the lipidlinked precursor oligosaccharide in the rough ER membrane Figure 12-52 Possible functions for the N-linked oligosaccharide chains? 1. Promoting protein folding & stability. 2. Protecting the protein from proteolysis. 3. Serving as a targeting determinant. 4. Facilitating or directing anterograde (forward) transport. 5. Promoting cell-to-cell adhesion. The role of N-linked glycosylation in ER protein folding Figure 12-53 The export & degradation of misfolded ER proteins * The ER functions as a quality control organelle. * Proteins that are not properly folded are exported from the ER and degraded in the cytosol. Figure 12-54 Quality control in the ER and Cystic Fibrosis * A particular deletion that removes three nucleotides in the Cftr gene is the most common mutation responsible for this disease. * * This deletion results in the removal of a phenylalanine residue, F508. * However, the encoded protein would be FUNCTIONAL if it was allowed to go to the plasma membrane. The encoded protein is recognized by the ER quality-control machinery and is ultimately targeted for degradation (in the cytoplasm). Plasma membrane M * Degradation Knowing the above, how might you try to treat CF patients that possess this cftr allele? Lipid synthesis in the ER * The cytoplasmic half of the ER bilayer is the primary site of phospholipid synthesis. The enzymes that catalyze these reactions are ER membrane proteins whose active sites face the cytosol. * Phospholipid translocators function to "flip" specific phospholipids from one half of the bilayer to the other. * Specific phospholipid transfer proteins (PLTPs) transport phospholipids from the ER to mitochondria and peroxisomes. Synthesis of phosphatidylcholine Figure 12-57 Molecular Biology of the Cell (© Garland Science 2008) The role of phospholipid translocators in lipid bilayer synthesis SCRAMBLASE FLIPPASE Figure 12-58 Phospholipid exchange/transfer proteins Ch. 13: Intracellular Vesicular Traffic Figure 13-2 Molecular Biology of the Cell (© Garland Science 2008) Biosynthetic-Secretory/Endocytic Pathway Figure 13-3 Molecular Biology of the Cell (© Garland Science 2008) Figure 13-3b Molecular Biology of the Cell (© Garland Science 2008) Vesicular Transport * The lumen of each compartment communicating by way of vesicular traffic is topologically equivalent. * The two primary pathways for vesicular traffic are known as the biosynthetic-secretory and the endocytic pathways. * A transport vesicle must select the cargo to be transported to the next compartment and exclude that which is to remain behind. * To ensure compartment identity, a transport vesicle must fuse only with the appropriate target organelle. Protein coats facilitate multiple steps of vesicular transport Coated Vesicles * Most transport vesicles form from specialized "coated" regions of the membrane and bud off as coated vesicles. * These coats are protein structures that form on the cytosolic face of a membrane region that will form the transport vesicle. * Several coat structures have been identified in eukaryotic cells and each appears to perform a distinct transport function. * Coated vesicles generally mediate the directional flow of specific types of membranes. * The assembly of a coat structure on a membrane may be the driving force in bud formation. * Coat proteins play an important role in the selection of vesicle cargo. Three examples of coated vesicles Figure 13-4 Molecular Biology of the Cell (© Garland Science 2008) Generation of membrane curvature a. Membrane deformation by proteins that exert mechanical force. b. Curvature generation by scaffolding proteins (coat proteins). c. Curvature generation by a hydrophobic insertion (wedging) mechanism. Different coated vesicles mediate distinct transport steps within the secretory pathway Figure 13-5 Molecular Biology of the Cell (© Garland Science 2008) Clathrin-coated Vesicles * The primary component of one membrane coat is clathrin, a large protein complex composed of three subunits each of a heavy chain and a light chain. * Clathrin-coated vesicles mediate Golgi-to-lysosome protein delivery and plasma membrane receptormediated endocytosis. * Clathrin coat assembly provides the mechanical force necessary for bud emergence and vesicle formation. * Other proteins in this coat, known as adaptins, mediate the binding and sequestration of specific transmembrane receptors and their bound cargo. Clathrin-coated pits & vesicles on the inner surface of the p.m. in cultured fibroblasts Figure 13-6 Molecular Biology of the Cell (© Garland Science 2008) The structure of a clathrin coat 2 3 Clathrin light chain Assembled clathrin 6 4 Clathrin heavy chain Clathrin triskelia 5 Figure 13-7 Clathrin coat assembly & disassembly Figure 13-8 Molecular Biology of the Cell (© Garland Science 2008) Ligand cargo 2 1 Receptor cargo Nucleation determinant 3 Clathrin light chain Adaptor Assembled clathrin 6 4 Clathrin heavy chain Clathrin triskelia 7 5 Uncoated vesicle Assembled clathrin at the plasma membrane Clathrin basket Figure 2 Receptor-mediated endocytosis and clathrin morphology. T he numbered sequence of events during receptor-mediated endocytosis is shown clockwise from upper left. , Adaptors (AP2) are recruited by a nucleation determinant, involving PtdIns(4,5)P 2 , in a process that requires cooperative interplay with clathrin and cargo (orange triangles and purple circles). Clathrin triskelia from the cytosol coassemble with adaptors and cargo, causing cargo sequestration and sorting. T rimerized clathrin heavy chains (red ) are based Cargo selection by the clathrin coat The role of dynamin in pinching off clathrincoated vesicles from the membrane. Figure 13-12 Molecular Biology of the Cell (© Garland Science 2008) Monomeric GTPases control coat assembly Figure 13-13a “Inactive” (GDP) GTP-binding proteins act as molecular switches (GTP) GEF = Guanine nucleotide Exchange Factor “Active” Figure 3-73 Molecular Biology of the Cell GAP = GTPase-Activating Protein Monomeric GTPases control coat assembly Sar1- COP II ARF - COP I Clathrin Figure 13-13a Coat recruitment & cargo selection Figure 13-13b Molecular Biology of the Cell (© Garland Science 2008) Formation of COPII-coated vesicles Sec13/31 “cage” Figure 13-13 How does a transport vesicle find its correct destination? Vesicle Targeting * To ensure compartment identity, transport vesicles must fuse only with the appropriate target membrane. * This specificity is mediated by two classes of proteins: the Rab family of monomeric GTPases and the transmembrane SNARE proteins that mediate membrane fusion. * The active GTP-bound Rab proteins interact with a diverse set of Ras effector proteins that mediate vesicle transport, tethering and fusion to the appropriate target membrane. * Membrane fusion is facilitated by the pairing of complementary transmembrane receptors present on the vesicle (v-SNAREs) and the target membrane (t-SNAREs). * SNARE complex disassembly after membrane fusion is catalyzed by NSF, a cytoplasmic ATPase. Tethering of a vesicle to a target membrane Figure 13-14 Molecular Biology of the Cell (© Garland Science 2008) The structure of a trans-SNARE complex Figure 13-16 Molecular Biology of the Cell (© Garland Science 2008) A model for how SNARE proteins may catalyze membrane fusion Figure 13-17 Molecular Biology of the Cell (© Garland Science 2008) NSF facilitates the dissociation of SNARE proteins after membrane fusion Figure 13-18 Molecular Biology of the Cell (© Garland Science 2008)