Hemoglobinopathies
advertisement

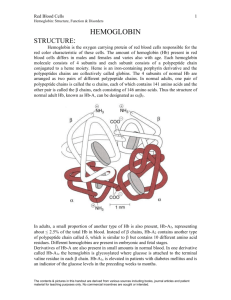
Dr.S.Chakravarty MD Specific Learning Objectives • At the end of today’s lecture you should be able to – – Enumerate the various hemoglobinopathies with their associated genetic defects . – Describe the molecular basis and the basis of laboratory diagnosis of Sickle cell anaemia , alpha and beta thalassemia in detail. α β β α 25% 25% 0.5% 1.5% 48% α α γ δ β α α γ δ β 25% 25% 0.5% 1.5% 48% Chromosome 16 Chromosome 11 α β α γ α δ β α γ α δ α HbA HbF HbA2 98% ~1% <3.5% Steps in globin chain synthesis: 1.Transcription 2.Modification of mRNA precursor by splicing 3.Translation by ribosomes & further modifications (i.e. glycosylation) • An inherited mutation of the globin genes leading to a qualitative or quantitative abnormality of globin synthesis. • Hereditary disorders that can result in moderate to severe anemia • • • • • Structural hemoglobinopathies Thallasemias Thallasemic Hb variants Hereditory persistance of fetal Hemoglobin Acquired hemoglobinopathies 10 1. Structural Hemoglobinopathies a) Altered Hb polymerization b) Altered affinity c) Unstable Hb Variants. 11 • Mutation : of Beta chain. is replaced by at 6th position • Polymerizes reversibly when deoxygenated to form a gelatinous network of fibrous polymers that stiffen the erythrocyte membrane. • The abnormal hemoglobin is less soluble under decreasing oxygen concentrations and polymerize into crystals that distort the red blood cells into a sickle shape. A number of factors may precipitate a sickle cell crisis, these include: •Hypoxia •Acidosis •Dehydration •Infections • Severe exercise •Increase physical / physiological demand (Pregnancy, physical exercise) Hb S is less soluble in acidosis and dehydration Hydrophobic valine causes stickiness Lippincott’s Illustrated Biochemistry 1. Hemolysis Anaemia 2. Occlusion of blood vessels by sickled red cellsSEVERE PAIN( DUE TO TISSUE ANOXIA) Site of Sickling Clinical Features Management Bone Painful crises Pain relief and hydration. Hydroxyurea Lung Acute chest syndrome Transfusion regimen, pain relief and hydration Brain Stroke Transfusion regimen. Heart Myocardial infarction Transfusion regimen, pain relief and hydration Spleen Acute splenic sequestration: Transfusion, pain relief and hydration Spleen Hyposplenism: Pneumovax • Complete Hemogram Anemia • Sickling test :- Blood smear prepared after adding reducing agent sodium dithionite See under microscope (NOT SPECIFIC ) • Solubility test :- Hemolysate is prepared in the presence of reducing agent .Opalescence is suggestive of sickle cells. (NOT SPECIFIC ) • Hb electrophoresis- CONFIRMATORY • Southern Blot -CONFIRMATORY • @ pH 8.6 Glutamic acid carboxy group is –ve charged • Lack of this charge of HbS makes it less negatively charged and decreases the electrophoretic mobility towards positive pole. • However at acidic pH (citrate buffer)HbS moves faster than Hb A Lippincott’s Illustrated Biochemistry • Repeated Blood transfusions- MAINSTAY • IRON OVERLOAD is a problem • CHELATION therapy with Desferrioxamine • CHRONIC HEINZ BODY ANAEMIAS -Unstable Hb variants have a tendency to denature. They tend to form molecular aggregates called Heinz Bodies within cells hemolysis – Hb Köln (Hb β 98valmet) – Hb Poole – γ chain unstable variant • INCREASED O2 AFFINITY – Hb Chesapeake – erythrocytosis (ODC shifts to left ) • DECREASED O2 AFFINITY – Hb Kansas – Cyanosis • Hb M– Most HbM are produced by substitution of Tyr for proximal/Distal His in the haem pocket of the alpha or ß-chainsThis results in facilitated oxidation of the hemoglobin to yield excess methemoglobin which leads to cyanosis. – Hb M Hyde Park, β92His→Tyr; Hb MBoston, α58His→Tyr; Hb MSaskatoon, β63His→Tyr; Hb MMilwaukee-1, β67Val→Glu. • Hereditary disorders that can result in moderate to severe anemia • Basic defect is reduced production of globin chains. •Found most frequently in the Mediterranean, Africa, Western and Southeast Asia, India and Burma •Distribution parallels that of Plasmodium falciparum Hb D Symbolism Alpha Thalassemia • Greek letter used to designate globin chain: Symbolism Alpha Thalassemia / : Indicates division between genes inherited from both parents: / • Each chromosome 16 carries 2 genes. Therefore the total complement of genes in an individual is 4 Symbolism Alpha Thalassemia - : Indicates a gene deletion: -/ Classification & Terminology Alpha Thalassemia • Normal / • Silent carrier -/ • Minor -/- --/ • Hb H disease --/- • Barts hydrops fetalis --/-- Symbolism Other Thalassemia • Greek letter used to designate globin chain: Symbolism Other Thalassemia + : Indicates diminished, but some production of globin chain by gene: + Symbolism Other Thalassemia 0 :Indicates no production of globin chain by gene: 0 Classification & Terminology Beta Thalassemia • Normal / • Minor /0 /+ • Intermedia 0/+ • Major 0/0 +/+ Some mutations lie within promoter regions and typically lead to reduced globin gene transcription. In some cases a single-nucleotide change in one of the exons leads to the formation of a termination, or "stop" codon, which interrupts translation of βglobin messenger RNA (mRNA) and completely prevents the synthesis of βglobin. Such alleles are designated β0. Mutations that lead to aberrant mRNA processing are the most common cause of β-thalassemia. Most of these affect introns, but some have been located within exons. If the mutation alters the normal splice junctions, splicing does not occur, and all of the mRNA formed is abnormal. Unspliced mRNA is degraded within the nucleus, and no β-globin is made Abnormal associations of otherwise normal subunits. • With severe α-thalassemia, the β-globin subunits begin to associate into groups of four (tetramers) due to the paucity of potential α -chain partners. • These tetramers of b-globin subunits are functionally inactive and do not transport oxygen. No comparable tetramers of alpha globin subunits form with severe beta-thalassemia. • Alpha subunits are rapidly degraded in the absence of a partner from the beta-globin gene cluster (gamma, delta, beta globin subunits). β-Thalassemias Thalassemia major β-thalassemia trait Homozygous or Severe, requires compound blood transfusions heterozygous (β0/β0, regularly β0/β+, or β+/β+) Defects in transcription, processing, or translation of mRNA, Asymptomatic, with resulting in absent mild microcytic (β0) or decreased anemia, or (β+) synthesis of βmicrocytosis without globin anemia β/β+or β/β0 α-Thalassemias Hydrops fetalis --/-- Fatal in utero HbH disease --/-α Moderately severe anemia α-thalassemia trait --/αα(Asian) or -α/--α(African) Similar to βthalassemia trait Silent carrier -α/αα Asymptomatic, normal red cells Gene deletions spanning one or both α-globin loci Excessive RBC BREAKDOWN • Anaemia • Bone changes (hair on end) • Ethnicity: Mediterranean, Africa, Southeast Asia • Hypo-Micro, Poikilocytosis • NRBC’s, reticulocytosis, basophilic stippling • Siderocytes (with repeated transfusions) Thalassemia Blood Smears X-ray of scull in Thalassemia: “Hair-on-end” MRI showing marked widening of the diploic space containing alternating bands (arrows) of hypointense trabeculae and hyperintense marrow. Beta thalassemia major Male 18 years HEPATOSPLENOMEGALY OVERALL DECREASED GROWTH • Hb Lepore: fusion seen in some types of thalassemia • Hb Constant Spring • chain with 31 additional amino acids • --/cs • Hereditary persistence of fetal hemoglobin (HPFH) This is usually caused by mutations in the β-globin gene. • Beneficial to patients with sickle cell or thalassemia. • Hb H • 4 tetramer and γ4 tetramers • Associated with --/- thalassemia • Hb Barts & hydrops fetalis • • • • Barts is a 4 tetramer Associated with --/-Lethal High concentrations are capable of sickling • Peripheral smear – • Severe cases present with • Microcytosis • Hypochromia • Poikilocytosis • Hb Electrophoresis • DNA studies (PCR + S.blot) • Time of presentation • Related to degree of severity • Usually in first few years of life • Untreated severe thalassemia • --/--: Prenatal or perinatal death • --/- & --/cs: Normal life span with chronic hemolytic anemia • Untreated thalassemia • Major: Death in first or second decade of life • Intermedia: Usually normal life span • Minor: Normal life span • Repeated Blood transfusions - MAINSTAY • Repeated blood transfusions leading to Iron excess and Hemosiderosis. • Pigment stones in Gall Bladder and CBD are more common in such patients. POINT Hb MUTATION POSITION AMINO ACID SUBSTITUTION Hb S β6 Glu- Val Hb C β6 Glu-Lys Homozygotes – CC Mild anaemia Heteozygous – AC no disease Double heterozygous SC –Moderate disease Hb E β26 Glu-Lys Heterozygous – Asymptomatic Homozygous – Mild disease Hb D (Punjab or Los Angeles) β121 Glu-Gln HbSD – severe disease Hb O (Arab) β121 Glu-Lys Homozygous –mild anaemia Hb G (Philadelphia) Hb Lepore α68 Glu-Lys δ(1-87) β(116-146) HbG Philadelphia is an α variant, often associated with deletions of the nonaffected a genes. With no deletions, there is approximately 20% HbG, with one deletion about 30% G is present, and with two about 40% is present. Lepore is the product of the indicated crossover during meiosis.(NOT A MUTATION) Secondary Laboratory Investigation Cellulose Acetate Hb Electrophoresis - A2/C Normal S F A+ Secondary Laboratory Investigation Cellulose Acetate Hb Electrophoresis - A2/C Normal Hb SS S F A + Secondary Laboratory Investigation Cellulose Acetate Hb Electrophoresis - A2/C Normal Hb SS Hb AS S F A + Secondary Laboratory Investigation Cellulose Acetate Hb Electrophoresis - A2/C Normal Hb SS Hb AS Hb SC Hb CC S F A + Can you identify which person has severe sickle cell anemia and who is heterozygous for the condition ? • In general on alkaline electrophoresis in order of increasing mobility are hemoglobins A2, E=O=C, G=D=S=Lepore, F, A, K, J, Bart's, N, I, and H. • In general on acid electrophoresis in order of increasing mobility are hemoglobins F,A=D=G=E=O=Lepore, S, and C • Test partners of heterozygous or affected individuals • Antenatal diagnosis from DNA obtained by chorionic villus sampling, or by amniocentesis MCQ 1 An Asian child has severe anemia with prominence of the forehead (frontal bossing) and cheeks. The red cell hemoglobin concentration is dramatically decreased, and it contains only beta-globin chains with virtual deficiency of alpha-globin chains. Which of the following mechanisms is the most likely explanation? A. A transcription factor regulating the alpha-globin gene is mutated B. A regulatory sequence element has been mutated adjacent to an alpha-globin gene C. A transcription factor regulating the beta-globin gene is mutated D. A transcription factor regulating the alpha and beta -globin genes is deficient E. A deletion has occurred surrounding an alpha-globin gene 67 MCQ 2 HbC disease is caused by a single amino acid substitution (lysine instead of Glutamic acid) at position 6 in the bete-globin chain of the hemoglobin molecule. Patients homozygous for HbC have a mild chronic hemolytic anemia. HbS disease generally causes a more severe condition compared to HbC disease because HbS disease: A. Impairs oxygen binding to the heme moiety B. Impairs proper folding of the alpha-helix in the beta-globin chain C. Allows hydrophobic interaction among hemoglobin molecules D. Impairs beta-globin interaction with 2,3-bisphosphoglycerate E. Stabilizes iron moiety at ferric state (fe3+). MCQ 3 An infant to a greek immigrant appears healthy at birth but develops transfusion dependent hemolytic anemia by the age of 6 months. his erythrocytes contain insoluble aggregates of hemoglobin subunits. The child developed normally in utero because at that time he produced high quantities of: A. Alpha globin B. Beta globin C. Gamma globin D. Delta globin E. Epsilon globin 69 MCQ 4 which one of the following statements concerning the ability of acidosis to precipitate a crisis in sickle cell disease is correct? A. acidosis influences the shape of hemoglobin B. Acidosis decreases the solubility of Hb S C. Acidosis favours the conversion of hemoglobin from the taut to the relaxed conformation D. Acidosis shifts the oxygen-dissociation curve to the left E. Acidosis decreases the ability of 2,3-BPG to bind to Hemoglobin.