Document
advertisement

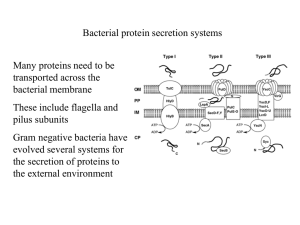
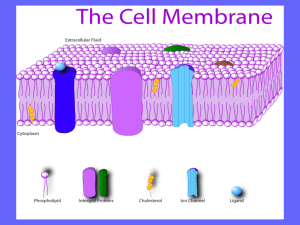
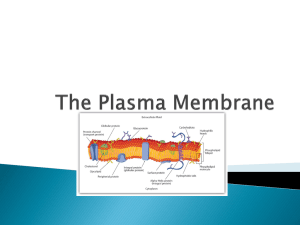
*توکسین های اثر کننده بر روی غشاء: *فسفولیپاز و لستیناز *توکسین های تشکیل دهنده ی پور در غشاء سلولی : توکسین هایی با ساختار (A plus B Subunit (:)A+B )Arrangement * The staphylococcal alpha-haemolysin pore (pink) is made up of seven subunits, which span the lipid bilayer as a beta-barrel, whereas ClyA (green) forms an iris-like barrel of alpha-helices from its twelve subunits Attachment and Entry of Toxins 1 - Direct entry 2 - Receptor-Mediated Endocytosis (RME) AB toxin enters cells via: 1) Receptor mediated endocytosis 2) Fusion of vesicle with lysosome 3) Acid environment of lysosome reduces disulfide bonds and releases A into cell 4) A has various cellular activities Figure 1. AB organization of bacterial toxins Diphtheria toxin is an AB toxin where the N terminal A domain (black) encodes an Enzyme activity, an ADP- ribosyl trasferase activity, NAD + EF-2 → ADP-r-EF-2 + nicotinamide + H+. The C-terminal B domain encodes a receptor binding function (grey) that binds to a grwoth factor receptor and enters cells through receptor-mediated endocytosis and a translocation function (white) which undergoes a pH-dependent conformation a change where chaafed amino acids are protonated which allows a pair of hydrophobic alpha helices to insert into the endosome membrane which is responsible for the delivery of A domain in to the host cytosol. Structure: PDB 1fol The plasma membranes of cells contain combinations of glycosphingolipids and protein receptors organized in glycolipoprotein microdomains termed lipid rafts. These specialized membrane microdomains compartmentalize cellular processes by serving as organizing centers for the assembly of signaling molecules, influencing membrane fluidity and membrane protein trafficking, and regulating neurotransmission and receptor trafficking. Lipid rafts are more ordered and tightly packed than the surrounding bilayer, but float freely in the membrane bilayer. Although more common in plasma membrane, lipid rafts have also been reported in other parts of the cell, such as Golgi and lysosomes Binding of superantigen SEC3 to TCR β Staphylococcus aureus superantigen SEC3 binds to the β chain of TCR through the hypervariable domain 4 (HV4), acting as a wedge to encourage TCR and MHCII interaction and activation lacking foreign peptide specificity. Arrow indicates HV4 loop on TCR β. Structure: PDB 1jck Comparison of antigen and superantigen binding to TCR : MHC class II complex. Left side: normal antigen presentation. Right side: superantigen stimulation in absence of antigen recognition. Red = MHC, blue = TCR, green = antigen, purple = superantigen MHC class II protein molecule MHC class I protein molecule Gram-negative - Type I secretion (ABC secretion) Properties: - ATP-binding cassette transporter (also in eukaryotes) - Single step traversal across CM and OM - Signal sequence at C-terminus - is not removed - ABC channel - 6-12 transmembrane helices - Accessory factor - bridges periplasmic space Post-translationally coordinated synthesistranslocation OM accessory factor P CM ATP ADP Genes fused or coordinately expressed on operon: N protein - GGXGSD ABC transporter C accessory factor (MFP) Outer membrane transport may not be linked Type I secreted proteins: RTX toxin (repeat in toxin) E. coli hemolysin bacteriocins metalloproteases Type I Secreted Toxins Proteins secreted via the Type I pathway contain glycine-rich repeats, usually at the C terminus, which bind calcium composed of a tripartite protein complex that includes an inner membrane transporter, a membrane fusion protein within the periplasm, and an outer membrane pore protein The adenylcyclase-hemolysin (CyaA) of Bordetella pertussis is a Type I secreted toxin . Entry into the host cell, via the alpha(M)beta(2) integrin (CD11b/CD18) cell receptor Upon entry into the cytosol of a host cell, CyaA is activated by the cofactor, calmodulin, and catalyzes the conversion of ATP to cAMP. Elevated intracellular cAMP disrupts signaling events within the cell and inhibits the ability of phagocytes to respond to B. pertussis infections Specifically, deregulation of cell signaling by CyaA affects protein kinase A, which modulates neutrophil migration, cytokine synthesis, oxidative bursts and organization of the actin cytoskeleton Type I Secreted Toxins OM accessory factor P CM ATP ADP N C Type II Secreted Toxins Type II secretion is facilitated by the Sec system and comprises protein complexes that span the bacterial inner and outer membranes Sec secretion includes three groups of proteins, a protein complex that spans the inner membrane, a periplasm-spanning protein complex, and outer membrane associated proteins. Type II secretion involves recognition of an N-terminal signal peptide on the nascent protein The signal sequence consists of N-terminal positive charged amino acids, internal hydrophobic amino acids, and a C-terminal domain with prolines and glycine One group of periplasmic proteins are homologous to pilin-like structures (known as pseudopilins) and regulatory proteins of the Type IV secretion system Type II Secreted Toxins Gram-negative - Type II secretion Sec-dependent secretory pathway signal peptidase leader peptide N ++ hydrophobic 1-5 7-15 C mature protein 3-7 Two step process: Step 1 - Transfer across cytoplasmic membrane - Leader (signal) peptide (18-26 aa) - SecA - binds leader (L), inserts in CM channel (requires ATP) - SecB - cytosolic chaperone (keeps unfolded) - SecYEG - CM channel complex SecY,E,G N L post-translational translocation SecB C (www.genome.ad.jp/kegg/ pathway/map/map03090.html) Type II Secreted Toxins Cholera toxin (CT) of Vibrio cholera is a Type II secreted toxin. CT is an AB5 toxin, where the A domain (~27.4 kDa) consists of two components, CT-A1 and CT-A2 and the B domain (~58 kDa) is a homopentameric protein complex CT-A1 ADP- ribosylates the Gα- subunit of the heterotrimeric protein, Gs. CT-A1 associates with the CT-A2 via a disulfide bond where CT-A2 inserts into the channel within the center of B5. The B5 domain binds specifically to the ganglioside, GM1a on the surface of intestinal epithelial cells [84]. Once bound, CT enters the cell through both clathrin -dependent and non- clathrin-dependent vesicle mechanisms involving lipid rafts Cytosolic CT-A1 binds A DP- ribosylation factor (ARF) and the activated CTA1 mono-ADP-ribosylates Arg 201 of Gsα, which blocks intrinsic GTPase activity and constitutively activates Gsα Gsα is a positive regulator of adenylate cyclase. Increased intracellular cAMP activates protein kinase A (PKA increasing active secretion of chloride ions Inhibition of the Na/K/2Cl co-transporter at the same time increases the unidirectional flow of chloride into the gut lumen, causing osmotic H20 flow into the gut lumen, the pathological outcome of cholera Type III Secreted Toxins The Type III secretion system (TTSS) is a bacterial virulence factor which injects cytotoxins (also termed effectors) in an unfolded or semi-folded state into a host cell. TTSS comprises inner and outer membrane protein complexes, but includes a hollow, pilin-like structure that extends beyond the outer membrane ring complex. The outer membrane structure is also similar to the Type II secretion system outer membrane ring and the Type IV pilus. Extracellular components of the TTSS include a needle, needle extension, and translocation pore, which deliver cytotoxins into the cytoplasm of host cells. The needle tip proteins appear to be adaptors that link the translocator proteins within the host membrane to the needle body for efficient transfer of cytotoxins The translocon protein complex prevents cytotoxin secretion before contact with the host cell by physically blocking the hollow channel of the needle complex Type III Secreted Toxins ExoS is a Type III secreted cytotoxin. ExoS is a 453 amino acid protein produced by Pseudomonas aeruginosa and is a dual function toxin, containing a Rho GTPase Activating Protein (Rho GAP) activity in the N terminus (96–219) and an ADP-ribosylation domain in the C terminus (234–453) ExoS Rho GAP domain increases γ-phosphate hydrolysis of GTP bound to Rho GTPases and in actives RhoA, Rac1, and Cdc42 Functional orgainzation of the type III cytotoxin Pseudomaons aeruginosa ExoS is a bi-functional toxins and is orgainzed inot discret functional doamisn (amino acids): secretion domain (1–15), chaperone binding domain (16–51), membrane localization domain (51–77), Rho GAP domain, active site residue R146 (96–243), and ADPribosyl transferase domain, active site residues E379, E381 (233–453) ADP-ribosylation is the addition of one or more ADP-ribose moieties to a protein . ADP-ribosylation is also responsible for the actions of some bacterial toxins, such as cholera toxin, diphtheria toxin, pertussis toxin, and heat-labile enterotoxin. These toxin proteins are ADP- ribosyl transferases that modify target proteins in human cells. For example, cholera toxin ADP- ribosylates G proteins, causing massive fluid secretion from the lining of the small intestine, resulting in lifethreatening diarrhea. P. aeruginosa ADP- ribosylates cytoskeleton and GTP-binding proteins ADP Ribosylation Factors (ARFs) are members of the ARF family of GTP-binding proteins of the Ras superfamily. ARF family proteins are ubiquitous in eukaryotic cells, and six highly conserved members of the family have been identified in mammalian cells. Although ARFs are soluble, they generally associate with membranes. They function as regulators of vesicular traffic and actin remodelling Pseudomonas aeruginosa produces exotoxin A (ETA) and four type III cytotoxins: ExoS, ExoT, ExoU and ExoY. Different clinical isolates of P. aeruginosa can express one or more of these four cytotoxins. The catalytic activity of each type III cytotoxin is activated by a host protein. ExoU is a recently described lipase that disrupts membrane function in mammalian cells. ExoY is an adenylate cyclase that elevates intracellular cyclic AMP (cAMP) to supraphysiological levels, which indirectly disrupts the actin cytoskeleton. ETA is the most potent protein toxin that P. aeruginosa secretes, and it inhibits mammalian protein synthesis by ADP- ribosylation of elongation factor 2 (EF2). One role of ExoS and ExoT is to disrupt the actin cytoskeleton through two independent enzymatic activities: Rho GTPase-activating protein (GAP) activity and ADP- ribosylation Gram-negative - Type IV secretion Properties - Used in export of protein complexes / DNA - Can translocate directly into host cell - Show homology to pilus-mediated conjugal transfer systems - Sec-like dependent translocation into periplasm - B11 - related to ATP-ases of type II system - D4 - DNA binding - may function in DNA transfer - B6, B7, B8 B9, B10 - core periplasmic components - B2, B5 - pilus components Bacteria that use type IV secretion: (H-J. Yeo, G. Waksman, J. Bacteriol. 2004) A. tumefaciens Agrobacterium tumefaciens - VirB-VirD Bordetella pertussis - pertussis toxin Helicobacter pylori - CagA Legionella pneumophila Type IV Secreted toxins The Type IV secretion system is a multi-functional protein complex, which transfers DNA between bacteria through conjugation (Type IVA), and transports effector proteins into host cells to regulate host responses to bacterial infection (Type IVB). The VirT IVA secretion system in Agrobacterium tumefaciens is the best characterized Type IV secretion system, which transfers DNA and proteins into plants to cause disease. DNA transfer requires a protein relaxase, which binds covalently to the 5′ end of ssDNA and causes secretion specifically in a 5′ to 3′ direction Type IV Secreted toxins Type V secretion system (T5SS) The Type V secretion system, also termed the autotransporter secretion group, includes 3 transport secretion mechanisms: termed Va, Vb, and Vc. Va group autotransporters are translated as a single protein composed of a N-terminal signal peptide sequence for transport by the Sec system, the effector domain, and a Cterminal outer membrane translocation domain Type V secretion system (T5SS) Type VI and Type VII Secretion Systems A type VI secretion system has been proposed in V. cholerae and P. aeruginosa . Type VI effectors do not possess N-terminal signal peptides and are Sec secretion independent, implicating a unique mechanism for effector transport relative to the Types I–V secretion systems. Recently, a secretion system was identified in the mycobacteria that was classified as the Type VII secretion for Gram-Positive bacteria Type I - ATP-binding cassette (ABC) transporter Type II - general pathway (Sec-dependent) - major secretory pathway Type III - contact-dependent translocation into eukaryotic cells Type IV - (Sec-like dependent) - translocation of DNA / protein complex Type V - auto-transporter (Sec-dependent) - includes b-pore forming domain Gram-negative secretion Sec-(or Sec-like) dependent Sec-independent Type I Type II Type III Type IV Type V host cell host cell OM P Sec B11 CM ATP ADP ATP ADP N C ATP ATP ADP N C Sec ADP N C (Adapted from Stathopoulus et al. (2000); provided by E Rucks)