Exome-seq Analysis
advertisement

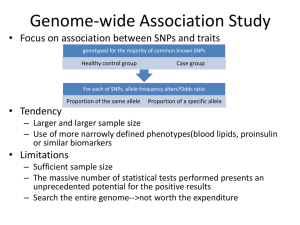
Exome Sequencing as Molecular Diagnostic Tool of Mendelian Diseases BIOS 6660 Hung-Chun (James) Yu Shaikh Lab 04/28/2014 Human Genetic Diseases Penetrance vs Frequency Kaiser J. Science (2012) 338:1016-1017. Human Genetic Diseases Complex Disorder • • • • Polygenic, many genes. Low penetrance/effect size. Multifactorial, environmental, dietary. Examples: heart disease, diabetes, obesity, autism, etc. Mendelian Disorder • • • Monogenic or polygenic. Full or high penetrance/effect size. Examples: sickle cell anemia and cystic fibrosis. Complex Diseases Multiple causes, and polygenic. Multiple genetics factors with low penetrance individually. Coronary artery disease Coriell Institute for Medical Research. https://cpmc1.coriell.org/genetic-education/diagnosis-versus-increased-risk Mendelian Diseases Veltman J.A. et al. Nat. Rev. Genet. (2012) 13:565-575. Mendelian Diseases Dominant Inheritance U.S. National Library of Medicine. http://ghr.nlm.nih.gov/ Mendelian Diseases Recessive Inheritance U.S. National Library of Medicine. http://ghr.nlm.nih.gov/ Exome Sequencing Bamshad, MJ., et al. Nat. Rev. Genet. (2011) 12:745-755. Exome Sequencing ~40Mb (coding) or 60Mb (coding + UTRs) Mendelian Diseases Identified by Exome Sequencing Timeline Gilissen C. et al., Genome Biol. (2011) 12:228. Mendelian Diseases Identified by Exome Sequencing By mid-2012, ~100 genes identified. By mid-2013, >150 genes identified. Rabbani, B., et al. (2012) J. Hum. Genet. 57:621-632. Types of Variation What kind of variation/mutation can be detected by Exome Sequencing? • • • SNV (single nucleotide variation) Small InDel, (insertion/deletion of <25bp) Large InDel, CNV (copy number variation) • Aneuploidy • Same as CNV Translocation • Possible, but not reliable. Possible, but not reliable. Limited. Complex rearrangement Not likely. Exome Variants SNV (single nucleotide variation) • • Synonymous: (1) Silent. Nonsynonymous: (1) Missense. (2) Nonsense. (3) Stop-loss. (4) Start-gain. (5) Start-loss. (6) Splice-site. http://upload.wikimedia.org/wikipedia/c ommons/6/69/Point_mutations-en.png http://www.webbooks.com/MoBio/Free/Ch5A4.htm Exome Variants Small InDel (insertion/deletion <25bp) Frameshift • In-frame • NHGRI Digital Media Database (DMD), http://www.genome.gov/dmd/ Variant and Population Frequency Novel/Private variant • Rare variant • Minor allele freq. (MAF) < 1%. Polymorphic variant • Never been reported before. MAF > 1% (0.01) or 5% (0.05). Databases dbSNP (NCBI): http://www.ncbi.nlm.nih.gov/SNP/ • 1000 Genomes: http://www.1000genomes.org/ • ESP (NHLBI): http://evs.gs.washington.edu/EVS/ • Exome Variants How to analyze enormous amount of variants in any given exome? Private/Novel Protein altering Coding + splice-site All Gilissen C. et al. Eur. J. Hum. Genet. (2012) 20:490-497. ~100 - 300 ~4,000 - 15,000 ~10,000 - 30,000 ~20,000 - 200,000 Exome Variants Bamshad, MJ., et al. Nat. Rev. Genet. (2011) 12:745-755. Exome Analysis Strategies Male Female Affected Heterozygous carrier Sex-linked heterozygous carrier Mating Consanguineous mating Gilissen C. et al., Eur. J. Hum. Genet. (2012) 20:490-497. Exome Analysis Strategies Linkage Large family with multiple affected individuals • Pathogenic variant co-segregate with disorder. • Homozygosity Affected patients from consanguine parents. • Homozygous mutation within a homozygous stretch in the genome. • Ideal for recessive disorders • Exome Analysis Strategies Candidate genes Biased approach • Require current biological knowledge • Good for screening or clinical diagnosis of known disorders. • Overlap Require multiple unrelated individuals with identical disorders. • Monogenic disorders • Exome Analysis Strategies De novo Sporadic mutation • Germline mutation during meiosis • Dominant inheritance • * Exome Analysis Strategies Double-hit Unaffected parents are heterozygous carries • Parental sequence info is very helpful • Recessive inheritance. • Homozygous Compound Heterozygous * # *# * * ** Trio-based Exome sequencing Family trio • Unaffected parents and an affected patient. Why we use trio? What can be tested using trio? Advantages? • Economical, efficient, single case required. Trio-based Exome sequencing Autosomal dominant De novo X-linked dominant De novo Autosomal recessive X-linked recessive Hemizygous in male Male Compound heterozygous Homozygous * Female Affected Heterozygous carrier Sex-linked heterozygous carrier XY * XY XX Trio-based Exome sequencing Candidate Genes/Variants Protein altering variants • Rare or novel variants • Variants that fit each inheritance model • Dominant Recessive Rare Variant Novel Variant De novo 0~1 0~1 Compound Heterozygous 0 ~ 20 0~3 Homozygous 0 ~ 20 0~3 X-linked 0 ~ 10 0~5 Case 1 Clinical information The patient was a 7-month-old boy when first evaluated. He was diagnosed with BPES by a pediatric ophthalmologist. In addition to blepharophimosis, ptosis, and epicanthus inversus normally associated with BPES, he had cryptorchidism, right hydrocele, wide-spaced nipples, and slight 2–3 syndactyly of toes. Clinical testing demonstrated a normal karyotype (46,XY), and normal FISH studies for 22q11.2 deletion, Cri-du-Chat (5p deletion) syndrome. Thyroid function was normal. Further, normal 7-dehydrocholesterol level was used to rule out Smith–Lemli–Opitz syndrome. Sanger sequencing and highresolution CNV analysis with Affymetrix SNP 500K arrays did not identify a FOXL2 mutation. Case 1 A-D: 2-month old. note blepharophimosis, ptosis, epicanthus inversus (A), posteriorly angulated ears with thickened superior helix and prominent antihelix (B), and slight 2–3 syndactyly of toes in addition to overlapping toes (C, D) E-F: 3.5-year old. Following oculoplastic surgery to correct ptosis; note right-sided preauricular ear pit (F, indicated by arrow). G-I: 12-year old. Note the recurrence of ptosis (L>R), arched eyebrows, abnormal ears, thin upper lip vermilion, small pointed chin, downsloping shoulders, and widespaced and low-set nipples. Case 2 Clinical information The proband is a nine year old girl who presented with microcephaly, unilateral retinal coloboma, bilateral optic nerve hypoplasia, nystagmus, seizures, gastroesophageal reflux, and developmental delay including not yet saying specific words (at 29 months old). On exam, she has microcephaly with a normal height, a down-turned upper lip, and fingertip pads. A karyotype and CGH analysis have been normal. Kabuki (KMT2D and KDM6A) and Angelman (UBE3A and MECP2) syndromes were suspected in this patient. Case 2 Case 3 Clinical information Case 3 was the result of a non-consanguineous union and he presented to care at four months of age with a seizure disorder, hypotonia and developmental delay. The patient underwent a left parietal craniotomy and partial resection of the frontal cortex without complete resolution of the seizure disorder. Initial laboratory studies included an elevated homocysteine and methylmalonic acid and a normal vitamin B12 level. Complementation analysis of the patient’s cell line placed the patient into the cblC class. Sequencing and deletion/duplication analysis (microarray) the MMACHC gene was negative in both skin fibroblasts and peripheral blood. Case 3 Feature Combined methylmalonic aciduria and homocystinuria. Severe developmental delay, infantile spasms, gyral cortical malformation, microcephaly, chorea, undescended testes, megacolon Case 3 Monster Max http://www.maxwatson.org/ Patient's older sister as a summer student in Shaikh Lab Data for Case Study 3 trios • • VCF files • • A total of 3 families/cases. Each family/case includes unaffected parents and an affected patient. Familial variants calls in VCF format, mapped to human GRCh37/hg19. 2x90bp paired-end reads, with ~50X coverage “Mini” Exome • • 100 genes with/without known disorder association. Validated causative genes, plus randomly selected genes. Exome NGS Workflow FASTQ 2x90bp BCF Filter based on Phred score, mapping quality, read depth, etc. SAM Filter unpaired, unmapped reads VCF BAM ? Filter PCR duplicates artifact BWA (Burrows-Wheeler Aligner) SAMtools VCF Format VCF (Variant Call Format) http://www.1000genomes.org/wiki/Analysis/Variant%20Call%20Format/vcf-variant-call-format-version-41 ## Meta-information lines FILTER, INFO, FORMAT # Header line VCF Format INFO AA : ancestral allele AC : allele count in genotypes, for each ALT allele, in the same order as listed AF : allele frequency for each ALT allele in the same order as listed: use this when estimated from primary data, not called genotypes AN : total number of alleles in called genotypes BQ : RMS base quality at this position CIGAR : cigar string describing how to align an alternate allele to the reference allele DB : dbSNP membership DP : combined depth across samples, e.g. DP=154 END : end position of the variant described in this record (for use with symbolic alleles) H2 : membership in hapmap2 H3 : membership in hapmap3 MQ : RMS mapping quality, e.g. MQ=52 MQ0 : Number of MAPQ == 0 reads covering this record NS : Number of samples with data SB : strand bias at this position SOMATIC : indicates that the record is a somatic mutation, for cancer genomics VALIDATED : validated by follow-up experiment 1000G : membership in 1000 Genomes VCF Format FORMAT GT: Genoetype. 0/0: Homozygous normal 0/1: Heterozygous variant 1/1: Homozygous variant PL: the Phred-scaled genotype likelihoods (>0). 0/0 0/1 1/1 174 ,0 ,178 GQ : Genotype quality (1-99) Question ?