Brooker Chapter 6
advertisement

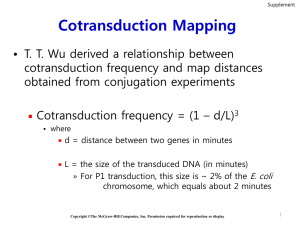
Bacterial Genetics Bacterial Genetics Bacteria are haploid identify loss-of-function mutations easier recessive mutations not masked Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-2 Bacterial Genetics Bacteria reproduce asexually Crosses not used genetic transfer bacterial DNA segments transferred Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-3 Genetic Transfer Enhances genetic diversity Types of transfer Conjugation Transduction direct physical contact & exchange phage Transformation uptake from environment Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-4 Conjugation Many, but not all, species can conjugate Only certain strains can be donors Donor strain cells contain plasmid called F factor + F strains Plasmid circular, extra-chromosomal DNA molecule F-factor Plasmid Genes for conjugation Conjugation Figure 6.4 Conjugation Figure 6.4 Conjugation Results of conjugation F factor plasmid may carry additional genes recipient cell acquires F factor converted from F– to F+ cell called F’ factors F’ factor transfer can introduce genes & alter recipients genotype Hfr Strains 1950s, Luca Cavalli-Sforza discovered E. coli strain very efficient at transferring chromosomal genes designated strain Hfr (high frequency of recombination) Hfr strains result from integration of F' factor into chromosome Figure 6.5a Hfr Conjugation Conjugation of Hfr & F– transfers portion of Hfr chromosome origin of transfer of integrated F factor takes 1.5-2 hrs for entire Hfr chromosome to be transfered starting point & direction of the transfer Only a portion of the Hfr chromosome gets into the F– cell F– cells does not become F+ or Hfr F– cell does acquire donor DNA recombines with homologous region on recipient chromosome Hfr Conjugation F– now lac+ pro– order of transfer is lac+ – pro+ F– now lac+ pro+ Figure 6.5b Interrupted Mating Technique Elie Wollman & François Jacob The rationale Hfr chromosome transferred linearly interruptions at different times various lengths transferred order of genes on chromosome deduced by interrupting transfer at various time Wollman & Jacob started the experiment with two E. coli strains Hfr strain (donor) genotype thr+ : Can synthesize threonine leu+ : Can synthesize leucine azis : Killed by azide tons : Can be infected by T1 phage lac+ : Can metabolize lactose gal+ : Can metabolize galactose strs : Killed by streptomycin F– strain (recipient) genotype thr– leu– azir tonr lac – gal – strr Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-21 Figure 6.6 Interpreting the Data Minutes that Bacterial Cells were Allowed to Mate Before Blender Treatment After 10 minutes, the thr+ leu+ genotype was obtained There were no surviving colonies after 5 minutes of mating Percent of Surviving Bacterial Colonies with the Following Genotypes thr+ leu+ azis tons lac+ gal+ 5 –– –– –– –– –– 10 100 12 3 0 0 15 100 70 31 0 0 20 100 88 71 12 0 25 100 92 80 28 0.6 30 100 90 75 36 5 40 100 90 75 38 20 50 100 91 78 42 27 60 100 91 78 42 27 The azis gene is transferred first It is followed by the tons gene The lac+ gene enters between 15 & 20 minutes The gal+ gene enters between 20 & 25 minutes Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-26 From these data, Wollman & Jacob constructed the following genetic map: They also identified various Hfr strains in which the origin of transfer had been integrated at different places in the chromosome Comparison of the order of genes among these strains, demonstrated that the E. coli chromosome is circular Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-27 The E. coli Chromosome Conjugation experiments have been used to map genes on the E. coli chromosome The E. coli genetic map is 100 minutes long Approximately the time it takes to transfer the complete chromosome in an Hfr mating Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-28 Arbitrarily assigned the starting point Units are minutes Refer to the relative time it takes for genes to first enter an F– recipient during a conjugation experiment Figure 6.7 Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-29 The distance between genes is determined by comparing their times of entry during an interrupted mating experiment The approximate time of entry is computed by extrapolating the time back to the origin Figure 6.7 Therefore these two genes are approximately 9 minutes apart along the E. coli chromosome Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-30 Transduction Transduction is the transfer of DNA from one bacterium to another via a bacteriophage A bacteriophage is a virus that specifically attacks bacterial cells It is composed of genetic material surrounded by a protein coat It can undergo two types of cycles Lytic Lysogenic Refer to Figure 6.9 Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-31 It will undergo the lytic cycle Prophage can exist in a dormant state for a long time Virulent phages only undergo a lytic cycle Figure 6.9 Temperate phages can follow both cycles Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-32 Plaques A plaque is a clear area on an otherwise opaque bacterial lawn on the agar surface of a petri dish It is caused by the lysis of bacterial cells as a result of the growth & reproduction of phages Figure 6.14 6-54 Transduction Any piece of bacterial DNA can be incorporated into the phage This type of transduction is termed generalized transduction Figure 6.10 Transformation Bacteria take up extracellular DNA Discovered by Frederick Griffith,1928, while working with strains of Streptococcus pneumoniae There are two types Natural transformation DNA uptake occurs without outside help Artificial transformation DNA uptake occurs with the help of special techniques Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display Transformation Natural transformation occurs in a wide variety of bacteria Bacteria able to take up DNA = competent carry genes encoding competence factors proteins that uptake DNA into bacterium & incorporate it into the chromosome A region of mismatch By DNA repair enzymes Figure 6.12 6-47 Transformation Sometimes, the DNA that enters the cell is not homologous to any genes on the chromosome It may be incorporated at a random site on the chromosome This process is termed nonhomologous recombination Like cotransduction, transformation mapping is used for genes that are relatively close together Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-48 Horizontal Gene Transfer Transfer of genes between different species vs Vertical gene transfer - transfer of genes from mother to daughter cell or from parents to offspring Sizable fraction of bacterial genes have moved by horizontal gene transfer Over 100 million years ~ 17% of E. coli & S. typhimurium genes have been shared by horizontal transfer Horizontal Gene Transfer Genes acquired by horizontal transfer Genes that confer the ability to cause disease Genes that confer antibiotic resistance Horizontal transfer has contributed to acquired antibiotic resistance 6.2 INTRAGENIC MAPPING IN BACTERIOPHAGES Viruses are not living However, they have unique biological structures & functions, & therefore have traits We will focus our attention on bacteriophage T4 Its genetic material contains several dozen genes These genes encode a variety of proteins needed for the viral cycle Refer to Figure 6.13 for the T4 structure Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-51 Contains the genetic material Figure 6.13 Used for attachment to the bacterial surface Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-52 In the 1950s, Seymour Benzer embarked on a ten-year study focusing on the function of the T4 genes He conducted a detailed type of genetic mapping known as intragenic or fine structure mapping The difference between intragenic & intergenic mapping is: 6-53 Plaques A plaque is a clear area on an otherwise opaque bacterial lawn on the agar surface of a petri dish It is caused by the lysis of bacterial cells as a result of the growth & reproduction of phages Figure 6.14 6-54 Some mutations in the phage’s genetic material can alter the ability of the phage to produce plaques Plaques are visible with the naked eye Thus, plaques can be viewed as traits of bacteriophages So mutations affecting them lend themselves to easier genetic analysis An example is a rapid-lysis mutant of bacteriophage T4, which forms unusually large plaques Refer to Figure 6.15 This mutant lyses bacterial cells more rapidly than do the wild-type phages Rapid-lysis mutant forms large, clearly defined plaques Wild-type phages produce smaller, fuzzy-edged plaques Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-55 Benzer studied one category of T4 phage mutant, designated rII (r stands for rapid lysis) It behaved differently in three different strains of E. coli In E. coli B rII phages produced unusually large plaques that had poor yields of bacteriophages In E. coli K12S rII phages produced normal plaques that gave good yields of phages In E. coli K12(l) (has phage lambda DNA integrated into its chromosome) The bacterium lyses so quickly that it does not have time to produce many new phages rII phages were not able to produce plaques at all As expected, the wild-type phage could infect all three strains Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-56 Complementation Tests Benzer collected many rII mutant strains that can form large plaques in E. coli B & none in E. coli K12(l) But, are the mutations in the same gene or in different genes? To answer this question, he conducted complementation experiments Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-57 Figure 6.16 shows the possible outcomes of complementation experiments involving plaque formation mutants Figure 6.16 Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-58 Benzer carefully considered the pattern of complementation & noncomplementation Benzer coined the term cistron to refer to the smallest genetic unit that gives a negative complementation test He determined that the rII mutations occurred in two different genes, which were termed rIIA & rIIB So, if two mutations occur in the same cistron, they cannot complement each other A cistron is equivalent to a gene However, it is not as commonly used Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-59 At an extremely low rate, two noncomplementing strains of viruses can produce an occasional viral plaque, if intragenic recombination has occurred rII mutations Viruses cannot form plaques in E. coli K12(l) rII mutations Viruses cannot form plaques in E. coli K12(l) Figure 6.17 Coinfection Function of protein A will be restored Therefore new phages can be made in E. coli K12(l) Viral plaques will now be formed Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-60 Figure 6.18 describes the general strategy for intragenic mapping of rII phage mutations 6-61 r103 r104 Take some of the phage preparation, dilute it greatly (10-8) & infect E. coli B Take some of the phage preparation, dilute it somewhat (10-6) & infect E. coli K12(l) Both rII mutants & wild-type phages can infect this strain Total number of phages 66 plaques rII mutants cannot infect this strain Number of wild-type phages produced by intragenic recombination 11 plaques 6-62 The data from Figure 6.18 can be used to estimate the distance between the two mutations in the same gene The phage preparation used to infect E. coli B was diluted by 108 (1:100,000,000) 1 ml of this dilution was used & 66 plaques were produced Therefore, the total number of phages in the original preparation is 66 X 108 = 6.6 X 109 or 6.6 billion phages per milliliter The phage preparation used to infect E. coli k12(l) was diluted by 106 (1:1,000,000) 1 ml of this dilution was used & 11 plaques were produced Therefore, the total number of wild-type phages is 11 X 106 or 11 million phages per milliliter Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-63 In this experiment, the intragenic recombination produces an equal number of recombinants Wild-type phages & double mutant phages However, only the wild-type phages are detected in the infection of E. coli k12(l) Therefore, the total number of recombinants is the number of wildtype phages multiplied by two Frequency of recombinants = Frequency of recombinants = 2 [wild-type plaques obtained in E. coli k12(l)] Total number of plaques obtained in E. coli B 2(11 X 106) 6.6 X 109 = 3.3 X 10–3 = 0.0033 In this example, there was approximately 3.3 recombinants per 1,000 phages Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-64 As in eukaryotic mapping, the frequency of recombinants can provide a measure of map distance along the bacteriophage chromosome The frequency of intragenic recombinants is correlated with the distance between the two mutations In this case the map distance is between two mutations in the same gene The farther apart they are the higher the frequency of recombinants Homoallelic mutations Mutations that happen to be located at exactly the same site in a gene They are not able to produce any wild-type recombinants So the map distance would be zero Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-65 Deletion Mapping Benzer used deletion mapping to localize many rII mutations to a fairly short region in gene A or gene B He utilized deletion strains of phage T4 Each is missing a known segment of the rIIA and/or rIIB genes Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-66 Let’s suppose that the goal is to know the approximate location of an rII mutation, such as r103 E. coli k12(l) is coinfected with r103 & a deletion strain If the deleted region includes the same region that contains the r103 mutation No intragenic wild-type recombinants are produced Therefore, plaques will not be formed If the deleted region does not overlap with the r103 mutation Intragenic wild-type recombinants can be produced And plaques will be formed Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-67 Figure 6.19 Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-68 As described in Figure 6.19, the first step in the deletion mapping strategy localized rII mutations to seven regions Other strains were used to eventually localize each rII mutation to one of 47 regions 36 in rIIA & 11 in rIIB At this point, pairwise coinfections were made between mutant strains that had been localized to the same region Six in rIIA & one in rIIB This would precisely map their location relative to each other This resulted in a fine structure map with depicting the locations of hundreds of different rII mutations Refer to Figure 6.20 Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-69 Contain many mutations at exactly the same site within the gene Figure 6.20 Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-70 Intragenic mapping studies were a pivotal achievement in our early understanding of gene structure Some scientists had envisioned a gene as being a particle-like entity that could not be further subdivided However, intragenic mapping revealed convincingly that this is not the case It showed that Mutations can occur at different parts within a single gene Intragenic crossing over can recombine these mutations, resulting in wild-type genes Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display 6-71