Microwave Spectroscopy
advertisement

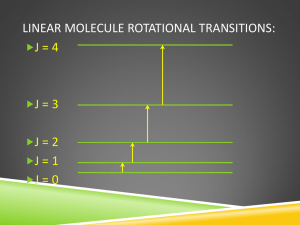
Microwave Spectroscopy or Rotational Spectroscopy Applied Chemistry Course: CHY101 Regions of Electromagnetic Radiation Frequency () Wavelength () Radio-waves Region Microwaves Region Infra-red Region Visible Region Ultra-violet Region X-ray Region -ray Region Frequency (HZ) 106 - 1010 1010 - 1012 1012 - 1014 1014 - 1015 1015 - 1016 1016 - 1018 1018- 1020 Wavelength 10m – 1 cm 1 cm – 100µm 100µm – 1µm 700 – 400 nm 400-10 nm 10nm – 100pm 100pm – 1 pm NMR, ESR Rotational Spectroscopy Vibrational spectroscopy Electronic Spectroscopy Electronic Spec. 0.001 – 10 J/mole Order of some 100 J/mole Some 104 J/mole Some 100 kJ/mole Some 100s kJ/mole 107- 109 J/mole 109- 1011 J/mole Energy UHF TV, cellular, telephones. (300 MHz and 3 GHz) FM Radio, VHF TV. AM Radio Microwave ovens, Police radar, satellite stations-- (3 to 30 GHz) Table lamp, Tube light (400 nm -800 nm or 400–790 THz) Sun Lamp X-ray Electromagnetic Radiation Electromagnetic Wave Regions of Electromagnetic Radiation The interaction of radiation with matter http://hyperphysics.phy-astr.gsu.edu/hbase/mod3.html Different types of Energy Connecting macroscopic thermodynamics to a molecular understanding requires that we understand how energy is distributed on a molecular level. ATOMS: The electrons: Electronic energy. Increase the energy of one (or more) electrons in the atom. Nuclear motion: Translational energy. The atom can move around (translate) in space. MOLECULES: The electrons: Electronic energy. Increase the energy of one (or more) electrons in the molecule. Nuclear motion: Translational energy. The entire molecule can translate in space. Vibrational energy. The nuclei can move relative to one another. Rotational energy. The entire molecule can rotate in space. The rotation spectrum of 12C16O at 40 K. The lines are nearly equally spaced and vary in intensity. We also will learn why the lines are nearly equally spaced and vary in intensity. Such spectra can be used to determine bond lengths, and even bond angles in polyatomic molecules. Absorption of Electromagnetic Radiation - The Coupling Mechanism An electromagnetic wave is an oscillating electrical field and interacts only with molecules that can undergo a change in dipole moment. The oscillating dipole can be provided by the rotation of a permanent dipole like for example HCl. This type of interaction leads to microwave spectra HCl Fig. The rotation of a polar diatomic molecule, showing the fluctuation in the dipole moment measured in a particular direction Microwave Spectroscopy Incident electromagnetic waves can excite the rotational levels of molecules provided they have an electric dipole moment. The electromagnetic field exerts a torque on the molecule. The spectra for rotational transitions of molecules is typically in the microwave region of the electromagnetic spectrum. Absorption of microwave radiation causes heating due to increased molecular rotational activity.... Microwave Spectroscopy Incident electromagnetic waves can excite the rotational levels of molecules provided they have an electric dipole moment. The electromagnetic field exerts a torque on the molecule. Homonuclear diatomic molecules (such as H2, O2, N2 , Cl2) – have zero dipole (non polar) -- have zero change of dipole during the rotation – hence NO interaction with radiation -- hence homonuclear diatomic molecules are microwave inactive Heteronuclear diatomic molecules (such as HCl, HF, CO) – have permanent dipolemoment (polar compound) -- change of dipole occurs during the rotation – hence interaction with radiation takes place – Therefore, heteronuclear diatomic molecules are microwave active. RIGID ROTOR For simplicity, we can consider only rotational motion of rigid diatomic molecule, A diatomic molecule can rotate around a vertical axis. The rotational energy is quantized. Assume a rigid (not elastic) bond r0 = r1 + r2 For rotation about center of gravity, C : m1r1 = m2r2 0 = m2 (r0 - r1) m2r0 r1 m1 m2 m1r0 r2 m1 m2 RIGID ROTOR Moment of inertia about C: IC = m1r12 + m2r22 = m2r2r1 + m1r1r2 = r1r2 (m1 + m2) m1m2 2 2 I r0 μr0 m1 m2 1 1 1 = reduced mass, μ m1 m2 A diatomic molecule can rotate around a vertical axis. The rotational energy is quantized. By the using Schrödinger equation, the rotational energy levels allowed to the rigid diatomic molecule are given by, J = Rotational quantum number (J = 0, 1, 2, …) I = Moment of inertia = mr2 = reduced mass = m1m2 / (m1 + m2) r = internuclear distance Energy is quantized Planck suggests that radiation (light, energy) can only come in quantized packets that are of size hν. Planck, 1900 E h Energy (J) Planck’s constant h = 6.626 × 10-34 J·s Frequency (s-1) Note that we can specify the energy by specifying any one of the following: 1. The frequency, n (units: Hz or s-1): 2. The wavelength, λ, (units: m or cm or mm): Recall: c 3. The wavenumber, 1 Recall: ~ ~ (units: cm-1 or m-1) E h E hc E hc~ Rotational Spectra of Rigid Diatomic molecule Rotational Energy Levels for rigid rotor: Where Rotational Spectra of Rigid Diatomic molecule For rigid rotor, J J + 1, The allowed rotational energy levels of a rigid diatomic molecule Allowed transitions between the energy levels of a rigid diatomic molecule and the spectrum Rotational Spectra of Rigid Rotor Apart from Specific rule, DJ 1, Gross rule- the molecule should have a permanent electric dipole moment, . Thus, homonuclear diatomic molecules do not have a pure rotational spectrum. Heteronuclear diatomic molecules do have rotational spectra. Selection Rule: Dj 1 Dj 1 (absorption) Dj 1 (emission) Rotational Energy levels Microwave Spectroscopy Incident electromagnetic waves can excite the rotational levels of molecules provided they have an electric dipole moment. The electromagnetic field exerts a torque on the molecule. Homonuclear diatomic molecules (such as H2, O2, N2 , Cl2) – have zero dipole (non polar) -- have zero change of dipole during the rotation – hence NO interaction with radiation -- hence homonuclear diatomic molecules are microwave inactive Heteronuclear diatomic molecules (such as HCl, HF, CO) – have permanent dipolemoment (polar compound) -- change of dipole occurs during the rotation – hence interaction with radiation takes place – Therefore, heteronuclear diatomic molecules are microwave active.