powerpoint

Lecture 34
Rotational spectroscopy: intensities
Rotational spectroscopy
In the previous lecture, we have considered the rotational energy levels.
In this lecture, we will focus more on selection rules and intensities.
Selection rules and intensities
(review)
Transition dipole moment m
=
ò
y
* f y i d t
Intensity of transition
I
µ
m 2
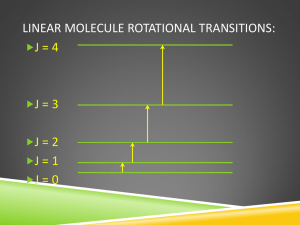
Rotational selection rules
Oscillating electric field (microwave)
Transition moment m fi
=
ò
e
=
ò
Y
J f f v
M f
J , f
Y
J f J , f
ˆ e
(
M
ò
e i v i
ˆ x e i v i i v i
Y
J i d t
M
J , i e d t d t v
) e d t
Y
J i v d t
M
J , i r d t r
µ permanent dipole m ev
= m ev
ò
Y
J f
M
J , f
ˆ Y
J i
M
J , i d t r
No electronic / vibrational transition
Rotational selection rules
Gross selection rule: nonzero permanent dipole
Does H
2
O have microwave spectra?
Yes
Does N
2
No have microwave spectra?
Does O
2
No have microwave spectra?
Quantum in nature
Microwave spectroscopy
How could astrochemists know H
2
O exist in interstellar medium?
Public image
NASA
Selection rules of atomic spectra(review)
TDM
µ
òò
Y
* l
¢ m l
¢
(
Y
10
or Y
1
±
1
)
Y lm l sin q d q d j ´ d m
¢ s m s
From the mathematical properties of spherical harmonics, this integral is zero unless
D m l
D l
= ¢ l
= ±
1
= m l
¢
D m s
m l
=
0
=
0,
±
1
Rotational selection rules
Specific selection rule:
D
D
J
= ±
1
M
J
=
0,
±
1 m fi
= m ev
ò
Y
J f
M
J , f
ˆ Y
J i
M
J , i d t r
Spherical & linear rotors
In units of wave number (cm
–1
):
( )
1
( )
(
2 BJ
Nonrigid rotor:
Centrifugal distortion
Diatomic molecule
( )
=
J
2
BJ J
+
1 1
2
D
J
»
4 B
3 n
2
Vibrational frequency
B
=
4 p cI
I
= m m
A m
B
A
+ m
B
R
2
Nonrigid rotor:
Centrifugal distortion
Nonrigid
D J
J
2
J
1
2
F J
Rigid
( )
=
BJ J
+
1
Appearance of rotational spectra
Rapidly increasing and then decreasing intensities
Transition moment 2
Degeneracy g
J
m
J
+
1, J
2 µ
2 J
1
J
2 J
1
1 m
2 ev
2
Boltzmann distribution
(temperature effect)
e
-
E
J
/ kT = e
hcBJ ( J
+
1)/ kT
Rotational Raman spectra
Gross selection rule: polarizability changes by rotation
Specific selection rule: x 2 + y 2 + z 2 ~ Y
0,0 xy , etc. are essentially Y
0,0
, Y
2,0
, Y
2, ± 1
, Y
2, ± 2
I
µ å k
ò
Y
J
* f
, M
J , f
ˆ Y k
E
0
(0)
(0) d t ò
-
E k
(0)
Y (0)* k
± h n
J i
, M
J , i d t
Linear rotors: Δ J = 0, ± 2
2
Spherical rotors: inactive (rotation cannot change the polarizability)
Rotational Raman spectra
Anti-Stokes wing slightly less intense than
Stokes wing – why?
Boltzmann distribution (temperature effect)
Rotational Raman spectra
Each wing ’ s envelope is explained by the competing effects of
Degeneracy
Boltzmann distribution (temperature effect)
H
2 rotational Raman spectra
Why does the intensity alternate?
H
2 rotational Raman spectra
Why does the intensity alternate?
Answer: odd J levels are triply degenerate
(triplets), whereas even J levels are singlets.
Nuclear spin statistics
Electrons play no role here; we are concerned with the rotational motion of nuclei.
The hydrogen’s nuclei (protons) are fermions and have α / β spins .
The rotational wave function (including nuclear spin part) must be antisymmetric with respect to interchange of the two nuclei.
The molecular rotation through 180 ° amounts to interchange .
Para and ortho H
2
Singlet ( paraH
2
)
Y r
1
, r
2
Sym.
( )
µ
{ spatial part of rotation
Antisym.
} { a
(1) b
(2)
b
(1) a
(2)
}
Nuclear (proton) spins
Triplet ( orthoH
2
)
Antisym.
Y
( )
µ
{ spatial part of rotation
}
¢
¢
Sym.
a
(1) a
(2) b
(1) b
(2) a
(1) b
(2)
+ b
(1) a
(2)
With respect to interchange (180 ° molecular rotation)
Spatial part of rotational wave function
By 180 degree rotation, the wave function changes sign as ( –1) J (cf. particle on a ring )
Para and ortho H
Singlet ( paraH
2
)
Y
2
( )
µ
Antisym.
{
J
= odd
}
¢
¢
Y r
1
, r
2
Sym.
( )
µ
{
J
= even
} { a
(1) b
(2)
b
(1) a
(2)
}
Sym.
a
(1) a
(2) b
(1) b
(2) a
(1) b
(2)
+ b
(1) a
(2)
Triplet ( orthoH
2
)
Summary
We have learned the gross and specific selection rules of rotational absorption and
Raman spectroscopies.
We have explained the typical appearance of rotational spectra where the temperature effect and degeneracy of states are important.
We have learned that nonrigid rotors exhibit the centrifugal distortion effects.
We have seen the striking effect of the antisymmetry of proton wave functions in the appearance of H
2 rotational Raman spectra.