Energy - uthgsbsmedphys.org
advertisement

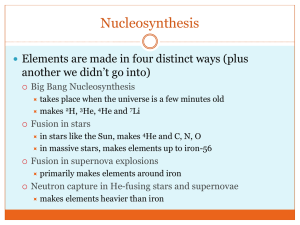
Neutron Interactions Part I Rebecca M. Howell, Ph.D. Radiation Physics rhowell@mdanderson.org B1.4580 November 2010 Why do we as Medical Physicists care about neutrons? • Neutrons in Radiation Therapy • Neutron Therapy • Contamination Neutrons on X-Ray Therapy • Contamination Neutrons in Proton Therapy • Unwanted patient dose • Shielding Considerations • Neutron Dose • The above will be discussed in lecture #2. • Today’s focus will be general neutron interactions Outline – Neutron Interactions • General properties • Neutron Reaction Cross Sections • Neutron Interactions General Properties • Neutrons are Neutral • Can Not interact by coulomb forces • Can travel through several cm of material without interacting. • Neutrons interact with nucleus of absorbing material (no not interact with orbital electrons). Reaction Cross Sections Used to describe neutron interaction probabilities. Reaction Cross Sections • A neutron reaction cross section quantitatively describes the probability of a particular interaction occurring between a neutron and matter. • When the reaction cross section is defined microscopicly on per nucleus, it is denoted by s and has S.I. Units = cm2 • Common unit for reaction neutron cross sections is the barn (10-24cm2). • Reaction cross sections are BOTH energy and interaction type dependent. Reaction Cross Sections • Energy and interaction type dependent - tabulated as a function of energy and interaction type. Reaction Cross Sections • Macroscopic cross section, S, probability per unit path length that a particular type of interaction will occur. S Ns • s = microscopic cross section, cm2 • N = number of nuclei per unit volume, nuclei/cm3 • All processes can be combined to calculate Stotal, probability per unit path length that any type of interaction will occur. STotal Sscatter Srad .capture ..... Exponential Attenuation • Neutrons are removed exponentially from a collimated neutron beam by absorbing material. I I oe Stotal t stotalN where Io I N = number of absorber atoms per cm3 (atomic density) s = the microscopic cross section for the absorber, cm2 t = the absorber thickness, cm Exponential Attenuation Example • In an experiment designed to measure the total cross section of lead for 10 MeV neutrons, it was found that a 1 cm thick lead absorber attenuated the neutron flux to 84.5% of its initial value. • The atomic weight of lead is 207.21, and its density is 11.3 g cm-3. • Calculate the total cross section from these data. Exponential Attenuation Example • Rearrange/Solve the general attenuation equation for s: I0 log I s Nt Calculate N, the atomic density of lead: 11.3g 6.031023 atoms 1 mole 3 cm mole 207.21g atoms 3.291022 cm3 log1.18 5.11024 cm2 5.1 barn 22 atoms 1cm 3.2910 3 cm Neutron Mean Free Path, l l 1 STotal Units: cm • Slow (low energy) neutrons • l is on the order of 1cm or less • For fast (high energy) neutrons • l may be tens of centimeters Neutron Mean Free Path, l Example • Calculate the mean free path for the previous example. s 5.110 cm 24 l 1 STotal 2 atoms N 3.29 10 cm 3 1 0.168cm sN 22 Some preliminary background compound nucleus model and resonance Compound Nucleus Model Multi-step Reaction • Incident neutron and target nucleus fuse together, then by successive nucleon-nucleon collisions within the combined system, the reaction energy becomes shared among many nucleons. A + a C • Eventually an equilibrium occurs and the compound nucleus exists in an excited state (10-16-10-18 seconds). A + a C* • Excitation is followed by deexcitation when a single nucleon or group of nucleons acquires enough energy to escape. A + a C B + b Compound Nucleus Model Multi-step Reaction The energy and nature of outgoing particles is determined by properties of the excited compound nucleus and NOT by the properties of the colliding particles from which it was formed. Compound Nucleus Model • Note: if the excitation energy is close to the threshold energy, the compound nucleus will decay by emitting only g-rays or the competing decay mode of internal conversion of electrons. • Recall: electron capture is a process that competes with g-ray emission in which the energy of an excited nuclear state is transferred to an atomic electron (typically K or L). Resonance • In this example, at ≈ 250 keV, the neutron energy is such that the compound nucleus 7Li is formed at an excitation which corresponds exactly to one of its higher states or natural frequencies. Peak is due to “resonance” in initial fusion process of the neutron with 6Li target. Resonance • At higher energies x-section may have large peaks. • Peaks = resonances • Occur at neutron energies where reactions with nuclei are enhanced • A resonance will occur if the energy of the incident neutron is close to the energy of an excited state of the compound nucleus Rinard, Fig. 12.3 Neutron Classifications and Interactions by energy Classification of Neutrons by Energy There are three energy categories of neutrons (NCRP-38): 1. Thermal neutrons are in thermal equilibrium with the medium they are in. The average energy of thermal neutrons is typically below 1eV, depending on temperature. The most probable velocity for thermal neutrons is 2200 meters per second at 20.44oC. This velocity corresponds to an energy of 0.0253eV. 2. Intermediate Energy Neutrons are classified as having intermediate energy range from above 1eV to tens of keV. 1. Fast Neutrons are classified as having energies above the intermediate neutrons. Classification of Neutrons by Energy The classification of neutrons by energy is somewhat dependent on the reference text. Some sources may include an epithermal category while others only include fast and slow (thermal). Category Fast Intermediate Epithermal Thermal Energy Range > 500 keV 10 keV – 500 keV 0.5 eV – 10 keV < 0.5 eV 0.5 eV Cd-cutoff energy: sharp drop occurs in Cd absorption cross section at 0.5 eV Neutron Interactions are Energy Dependent Overview of Neutron Interactions Scatter and Absorption Total Scatter Elastic Inelastic Scatter Scatter (n,n) (n,n’) Absorption Nonelastic Processes (n,n’3a) Sometimes shown as (n,ng) Also called “neutron capture” (n,n’4a) (n,n’etc) Electro- Charged magnetic (n,p) (n,g) (n,a) (n,d) (n,etc) Sometimes called “radiative” capture Neutral Fission (n,f) (n,2n) (n,3n) (n,4n) (n,xn) Sometimes called “transmutation” Boxes shaded in light blue follow the compound nucleus model. General Neutron Interactions Scattering and Absorption Scatter Absorption • When neutron is elastically or • When neutron is absorbed by inelastically scattered by nucleus nucleus, a wide range of radiations speed and direction change, but can be emitted or fission can be nucleus is left with same number induced. of protons and neutrons as before the interaction. • Different the number of protons and/or neutrons than before the interaction. • Elastic Scatter (n,n) • Inelastic Scatter (n,n’) Electromagnetic Neutral • (n,g) • • • • Charged • • • • (n,p) (n,a) (n,d) (n,etc) (n,2n) (n,3n) (n,4n) (n,etc) Fission • (n,f) Neutron Interactions are Energy Dependent • Fast neutrons are most likely to undergo scatter interactions with atoms in their environment. • Elastic Scatter – dominate for lower energy fast neutrons • Inelastic Scatter - above 1-Mev • Lower energy neutrons (thermal or near thermal) are likely to undergo absorption reactions with atoms in their environment. Neutron Scatter • Elastic Scatter – Kinetic Energy Conserved • More likely in low Z materials • More likely at lower energies, < 1MeV • Maximum amount of energy that can be lost is function of target nuclei mass. • Larger cross sections • Inelastic Scatter – Kinetic Energy NOT Conserved. • • • • • More likely in high Z materials More likely at higher energies E > 1MeV Can loose large amounts of energy in one collision Smaller cross sections Threshold Energy Neutron Elastic Scatter (n,n) • Elastic scattering is the most likely interaction between (lower energy) fast neutrons and low Z absorbers. • Billiard ball type collision • • Direct (head-on) collision – More energy transferred Indirect (grazing) Collision – Less Energy transferred • Kinetic energy and momentum are conserved • Light recoiling nucleus can cause high LET tracks Kinematics of Neutron Elastic Scattering • For incoming neutrons conservation of energy and momentum in the center-of-mass coordinate system gives the following relation for energy of the recoil nucleus: • Convert to laboratory system (general target nucleus is at rest): • Recoil nucleus energy in terms of its own angle of recoil. 2A 1 cosEn ER 2 1 A 4A 2 ER cos En 2 1 A Knoll fig 15-12 Note: assume incoming neutrons have nonrelativistic kinetic energy (En<939MeV), Definition of Symbols • A= mass of target nucleus (laboratory system) • En = incoming neutron kinetic energy (laboratory system) • ER = recoil nucleus kinetic energy (laboratory system) • scattering angle of the recoiled neutron in the center-ofmass coordinate system • scattering angle of the recoiled neutron in the lab coordinate system Kinematics of Neutron Elastic Scattering • Equation demonstrates that energy given to recoil nucleus is determined by scattering angle: 4A 2 ER cos En 2 1 A Elastic Scatter Grazing Angle Encounter • For grazing angle encounter, the neutron is only slightly deflected and the recoil target nucleus is emitted almost perpendicular to the incident neutron, ≈90. 0 • Energy of recoil nucleus : 4( A) 2 ER cos 90 En 2 1 A • For a grazing hit almost no energy goes to recoil nucleus, regardless of mass of the target nuclei. Elastic Scatter Direct Head-On Encounter • For head-on direct collision between an incoming neutron and a target nucleus, the recoil is emitted in almost the same direction as the incident neutron, ≈0. • Energy of recoil nucleus : 1 4( A) 2 ER cos 0 En 2 1 A • For a direct hit, energy that goes to recoil nucleus, depends on mass of the target nuclei. ER 4( A) 2 En 1 A Maximum Fractional Energy Transfer in Neutron Elastic Scattering Target ER max 4( A) 2 E 1 A Nucleus n 1H 1 2H 8/9=0.889 3He 3/4=0.750 4He 16/25=0.640 12C 48/169=0.284 16O 64/289=0.221 For direct head-on collisions: • The maximum fractional energy transfer increases as the mass of target nuclei decreases: • Nuclei with lower mass are more effective on a “per collision” basis for slowing down neutrons! Energy Distribution of Recoil Nuclei (from Elastic Neutron Scatter) • All scattering angles are allowed. • However, for most target nuclei, forward and backward scattering are somewhat favored. • Actual energy distribution for recoil nuclei is a continuum between the two extremes. Neutron Inelastic Scatter (n,n’ or (n,ng) • Inelastic Scatter - neutron is captured by target nucleus and is reemitted (may not be same neutron) along with g-ray. Inelastic scatter follows the compound nucleus model: 1. Neutron collides with nucleus and fuse together to form a combined system. 2. By successive nucleon-nucleon collisions within the combined system, the reaction energy becomes shared among many nucleons 3. Eventually an equilibrium occurs and the compound nucleus exists in an excited state. 4. Excitation energy is emitted as gamma photon, g can have substantial energy. 5. Neutron (not necessarily the incoming neutron) is emitted. Neutron Inelastic Scatter • Inelastic Scatter = Threshold Phenomenon • Infinite threshold for H (inelastic can not occur) • 6 MeV Threshold for O • 1 MeV Threshold for Ur • Cross section increases with increasing energy. • s ≤1 barn for low Energy neutrons. • s approaches geometric cross-section of target nucleus at high energies i.e. inelastic scatter is dominate interaction mechanism at higher energies. General Neutron Interactions Nonelastic Processes (n,n’3a) (n,n’4a) (n,n’etc) Nonelastic Processes • Similar to inelastic scatter in that the process follows a compound nucleus model and that there is a recoil neutron. • Different from inelastic scatter because instead of emitting grays, additional secondary particles can be emitted (in addition to scattered neutron). • Nucleus has different number of p+ and no after interaction. • Different from absorption because neutron is not absorbed, a scattered neutron is emitted (may not be the same one that entered reaction). • Sometimes called nonelastic scatter. Nonelastic verses Inelastic • Both non-elastic and inelastic scatter follow a compound nucleus model. • Whether the compound nucleus will deexcite via non-elastic or inelastic scatter is determined by the energy of the incident neutron….. if the energy of the incident neutron is very close to the threshold energy, de-excitation occurs by emission of gamma rays rather than by additional particle emissions i.e. inelastic scatter is favored over non-elastic scatter. Absorption (Neutron Capture) • Low energy neutrons (thermal or near thermal) are likely to undergo absorption reactions. • In this energy range, the absorption cross-section of many nuclei, has been found to be inversely proportional to the square root of the energy of the neutron. • one-over-v law for slow neutron absorption 1 1 s E Thermal Neutron Absorption Cember fig 5.23 Thermal Neutron Absorption Cross Sections Halflife Cross section [barn atom-1] Isotope Abundance Isotope Produced 23Na 100% 24Na 15 h 0.93 31P 100% 32P 14.3 d 0.18 41K 6.9% 42K 12.4 h 1.46 58Fe 0.33% 59Fe 45.1 d 1.15 59Co 100% 60Co 5.26 y 37 197Au 100% 198Au 2.69 d 99 10B 19.8% 7Li Stable 3837 B (all Cd (all isotopes) • If the cross section at E0 is s0, then the cross section for any other neutron (within the validity of the 1/v law is given by: 759 isotopes) 113Cd • Thermal neutron cross sections are given for neutrons whose energy is 0.025eV. 12.3% 114Cd Stable 20000 2450 E0 s 0 s0 E Neutron Activation • Neutron activation is the production of a radioactive isotope by absorption of a neutron. • Activation reactions follow absorption reactions. Examples: • 14N(n,p)14C • 10B(n,a)7Li • 113Cd(n,g)114C Activation Good and Bad • Byproducts of activation can have substantial energy: • Good: These byproducts can be measured. This technique is one of the methods most frequently used for neutron detection. • Detection class: We will discuss neutron detection via activation foils. • Bad: These byproducts can pose a radiation hazard. • Must be considered in neutron shielding design. “Most Common” Neutron Interactions in Tissue Neutron Interactions with Tissue • The type of interaction and the amount of dose deposited in the body is strongly dependent on neutron energy and absorbing material. • The most common elements in the human body are Hydrogen, Carbon, Nitrogen, and Oxygen. • Neutrons are indirectly ionizing and but give rise to densely ionizing (high LET) particles: recoil protons, a-particles, and heavier nuclear fragments • These particles then deposit dose in tissue. Fast Neutron Interactions in Tissue • Higher energy neutrons interact with carbon and oxygen via nonelastic processes and result in the release of charged a-particles, (n,n’3a) and (n,n’4a). • These a-particles then deliver dose to tissue Fast Neutron Interactions in Tissue • Recoil a-particles A neutron interacts with a Carbon A neutron interacts with an Oxygen nucleus (6 protons and 6 neutrons), nucleus (8 protons and 8 neutrons) resulting in three a-particles. , resulting in four a-particles. (Hall, Fig 1.10) (Hall, Fig 1.10) Intermediate Neutron Interactions in Tissue • Intermediate energy neutrons primarily interact with hydrogen nuclei via elastic scatter. • Dominant mechanism of energy transfer in soft tissues 3 Reasons 1. Hydrogen is the most abundant atom in tissue. 2. A proton and a neutron have similar mass, 938 MeV/cm2 versus 940 MeV/cm2. 3. Hydrogen has a large elastic scatter cross-section for neutrons. Thermal Neutron Interactions in Tissue • Absorption is the dominant interaction mechanism for thermal neutrons in tissue. • Absorption is followed by activation. • Activation decay products deliver dose to tissue. Thermal Neutron Interactions in Tissue • The major component of dose from thermal neutrons is a consequence of the 14N(n,p)14C + 0.62 MeV • 0.04 MeV to recoil nucleus (local absorption) • 0.58 MeV to proton (range of ~10-6 m local absorption) • Dominant energy transfer mechanism in thermal and epithermal region in body • Kerma = dose • Another thermal neutron interaction of some consequence is the 1H(n,g)2H + 2.2 MeV • 2.2 MeV to gamma (nonlocal absorption) • Small amount of energy to deuterium recoil (local absorption) • Kerma dose (non-local absorption) Summery Neutron Interactions with Tissue The amount of dose deposited in the body is strongly dependent on neutron energy. • Fast neutrons interact with carbon and oxygen via nonelastic processes and result in the release of charged a-particles, (n,n’3a) and (n,n’4a). These a-particles then deposits dose to tissue. • Intermediate energy neutrons primarily interact with hydrogen nuclei via elastic scatter. The recoil proton then deposits dose in tissue. • Absorption is the dominant interaction mechanism for thermal neutrons in tissue and is followed by activation. The major component of dose from thermal neutrons is a consequence of the 14N(n,p)14C which results in a 0.58 MeV proton. References/Acknowledgements • Glenn Knoll. Radiation Detection and Measurement, 4th Ed. (2010) • Herman Cember. Introduction to Health Physics 3rd Ed. (1996) • Eric J. Hall. Radiobiology for the Radiologist 5th Ed. (2000) • Frank H. Attix. Introduction to Radiological Physics and Radiation Dosimetry. (1986)