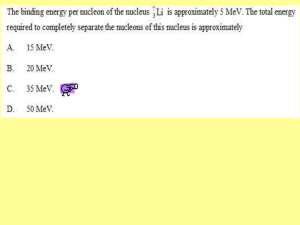Mass defect and binding energy
advertisement

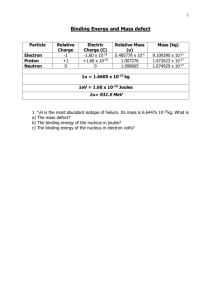
Topic – Physics 2a Mass defect and binding energy • • • • Prior learning Atomic structure Electrical forces Key words – Atomic nucleus,mass difference, mass of products, nuclear reactions, total mass Topic – Physics 2a By the end of this lesson we should be able to: • Know how binding energy is linked to Einstein's equation – E=mc2 • Calculate mass difference • Complete problem solving questions on binding energy Topic – Physics 2a What is binding energy? • Nuclei are made up of protons and neutron, but the mass of a nucleus is always less than the sum of the individual masses of the protons and neutrons which constitute it. • The difference (missing mass) is a measure of the nuclear binding energy which holds the nucleus together. • This binding energy can be calculated from the Einstein relationship: • Nuclear binding energy = Δmc2 Topic – Physics 2a Mass defect • The measured mass of a nucleus is always less than the sum of the individual masses of its nucleons. • This difference is called MASS DEFECT Topic – Physics 2a Binding energy and mass defect • The mass defect is a measure of the atoms binding energy • The more binding energy per nucleon the greater the atoms stability – held together stronger!! Topic – Physics 2a To calculate binding energy of a nucleus… • Add the mass of the individual nucleons (protons and neutrons) • Then subtract the mass of the actual atom itself • The mass left over – Mass defect Topic – Physics 2a Values and Equation • • • • Mass of proton 1.67262 x 10-27kg or 1.00727u Mass of neutron 1.67493 x 10-27 kg or 1.00867u Mass of electron 9.11 x 10-31kg or 5.49 x 10-4u See tables/data sheet for other values of atoms • Mass difference = mass of particles– mass of atom Topic – Physics 2a Example: When a neutron combines with a proton Deuteron nucleus • Neutron mass = 1.00867u • Proton mass = 1.00728u • = 2.01595u • Deuterium nuclear mass = 2.01350u • 2.01595 – 2.01350 = • Difference (loss) = 0.00245u Topic – Physics 2a Converting into energy • • • • • Constants: 1eV = 1.6 x 10-19J 1 MeV = 1.6 x 10-13 J 1 u = 1.6606 x 10-27 kg 1u = 931 MeV • speed of light, c = 3.0 x 108 ms-1 Topic – Physics 2a Example: converting to energy • Mass defect is then converted into an energy equivalent • x 931 • 0.00245u x 931 = 2.3MeV of energy • This is the energy that would be required to pull the nucleus apart Topic – Physics 2a Worksheet • Eb = md c2 where: • Eb = the binding energy in joules (J) • md = mass defect in kg. • c = speed of light, 3.0 x 108 ms-1 Topic – Physics 2a Example- Calculate the binding energy of a lithium-7 nucleus, in joules and MeV. • mass defect = (mass of neutrons and protons) - (mass of nucleus) = [(3 x mass proton) + (4 x mass neutron)] - (mass of lithium ) In kg = [(3 x 1.67 x 10-27) + (4 x 1.68 x 10-27)] - 1.165 x 10-26 = 5.01 x 10-27 + 6.72 x 10-27 - 1.165 x 10-26 = 8.0 x 10-29 kg E = Mc2 E = 8.0 x 10-29 x (3.0 x 108)2 = 7.12 x 10-12 J In u units 1 u = 1.6606 x 10-27 kg therefore 8.0 x 10-29 / 1.66 x 10-27 = 0.4818 u 0.4818 x 931 = 44.86MeV (check that’s correct by x 1.6 x 10-13 to get back into J) Topic – Physics 2a In the sun, hydrogen atoms combine to make helium in the process of fusion. In this process, energy is released. One of the reactions is shown. Determine the binding energy release for this reaction in joules and MeV. 1 1 2 1 H = 1.67 x 10-27 kg H = 3.34 x 10-27 kg md = (mass 11H + 21H ) - (mass 3 2 He = 5.00 x 10-27 kg = 1.00 x 10-29 kg 3 2 He ) = (1.67 x 10-27 + 3.34 x 10-27) – 5.00 x 10 -27 In joules: In MeV Eb = md x c2 = 1.00 x 10-29 x (3.0 x 108)2 Eb = 9.00 x 10-13 J mass in u = 1.00 x10 -29 1.66 x10 - 27 = 6.024 x 10-3 u MeV = 6.024 x 10-3 x 931 = 5.61 MeV Topic – Physics 2a A common reaction in nuclear fission power 235 1 141 92 1 U + n ® Ba + Kr + 3 plants is , 92 0 56 36 0n calculate the energy released from this reaction in joules and MeV. 25 235 92 U = 3.90 x 10 kg -25 141 = 2.34 x 10 kg Ba 56 -25 92 = 1.53 x 10 kg Kr 36 92 1 235 1 md = 141 56 Ba + 36 Kr + 3 0 n 92 U + 0 n = (2.34 x 10-25 + 1.53 x 10-25 + [3 x 1.68 x 10-27]) - (3.90 x 10-25 +1.68 x 10 -27 kg) = 3.9204 x 10-25 - 3.9168 x 10 -25 = 3.6 x 10-28 J md in u = 3.6 x10 -28 1.66 x10 - 27 MeV = 0.2169 x 931 = 202 MeV = 0.2169 u 1 u = 1.6606 x 10-27 kg therefore Topic – Physics 2a Extension Questions 1. What is the binding energy per nucleon in MeV for the following atoms involved in nuclear energy: • a. U-238 nucleus b. He-4 nucleus c. Fe-56 (one of the most stable atoms) (Fe = 55.934939 u) 2. The oxygen atom O has an isotope, O. Find the binding energy of each nucleus and thus • determine which is more stable. 3. Calculate the energy released from one decay of U-238. Topic – Physics 2a 1. What is the binding energy per nucleon in MeV for the following atoms involved in nuclear energy: a. U-238 nucleus U-238 = 92p + 146n md = ([92 x 1.00783) + (146 x 1.00867)] - U-238 = 92.72036 + 147.26582 – 238.05079 = 1.93539 m = 1.93539 x 1.6606 x 10-27 = 3.2130 x 10-27 J E = 3.2130 x 10-27 x (3 x 108)2 = 2.8925 x 10-10 J per atom as there are 238 nucleons, then binding energy per nucleon = 2.8925x10 -10 = 1.21 x 10-12 J 238 b. He-4 nucleus He-4 = 2p + 2n = (2 x 1.00783) + (2 x 1.00867) – 4.00260 = 0.0304 m = 0.0304 x 1.6606 x 10-27 = 5.04822 x 10-29 J E = 5.04822 x 10-29 x (3 x 108)2 = 4.5434 x 10-12 J per atom binding energy per nucleon = 4.5434x10 - 12 = 1.136 x 10-12 J 4 Topic – Physics 2a c. Fe-56 (one of the most stable atoms) (Fe = 55.934939 u) Fe-56 = 26p + 30n = (26 x 1.00783) + (30 x 1.00867) – 55.934939 = 0.528741 m = 8.78027 x 1028 kg E = 8.78027 x 10-28 x (3 x 108)2 = 7.90225 x 10-11 7.90225 x10 -11 binding energy per nucleon = = 1.41 x 10-12 J 56 You will notice that the binding energy per nucleon is greater than the previous two examples. Topic – Physics 2a 2. The oxygen atom 168 O has an isotope, and thus determine which is more stable. 17 8 O. Find the binding energy of each nucleus O-16 = 8p + 8n = (8 x 1.00783) + (8 x 1.00867) – 15.99943 = 0.13257 m = 2.20146 x 1028 kg E = 2.20146 x 10-28 x (3 x 108)2 = 1.9813 x 10-11 1.9813x10 - 11 binding energy per nucleon = = 1.238 x 10 -12 J 16 O-17 = 8p + 9n = (8 x 1.00783) + (9 x 1.00867) – 16.99913 = 0.14154 m = 2.3504 x 1028 kg E = 2.3504 x 10-28 x (3 x 108)2 = 1.1154 x 10-11 2.1154x10 -11 binding energy per nucleon = = 1.244 x 10 -12 J 17 Oxygen 17 has the greater binding energy so is more stable. Topic – Physics 2a 3. Calculate the energy released from one decay of U-238. 238 92 U® 42 He+ 23490Th md = 238.05079 – (4.00260 + 234.04360) = 4.59 x 10-3 u = 7.622154 x 10-30 kg E = mc2 = 7.622154 x 10-30 x (3 x 108)2 = 6.86 x 10 -13 J and 6.86 x10 - 13 1.6 x10 - 13 = 4.29 MeV Topic – Physics 2a links • http://www.s-cool.co.uk/alevel/physics/nuclear-energy/reviseit/mass-defect • http://www.colorado.edu/physics/200 0/periodic_table/amu.html • http://www.alevelphysicstutor.com/we-nucbinding-energy.php Topic – Physics 2a By the end of this lesson we should be able to: • Know how binding energy is linked to Einstein's equation – E=mc2 • Calculate mass difference • Complete problem solving questions on binding energy