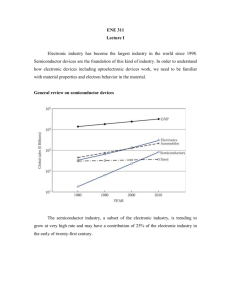
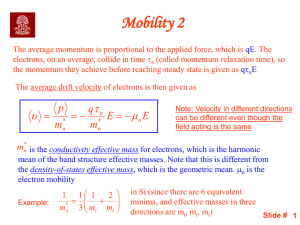
Lecture 1 - web page for staff
advertisement

ENE 311 Lecture 1 Syllabus • Instructor: Apichai Bhatranand • E-mail: apichai.bha@kmutt.ac.th • Phone: 02-470-9063 • Office: CB 40903 • Office Hours: By appointment. Walk-in is also welcomed. Syllabus • Textbook: “Semiconductor Devices Physics and Technology”, S. M. Sze, Wiley. • References: − “Lectures on the Electrical Properties of Materials”, L. Solymar and D. Walsh, Oxford. − “Solid State Electronic Devices”, B. G. Streetman, Prentice Hall. − “Microelectronic Devices”, E. S. Yang, McGrawHill. Syllabus • Grading: Mid Term Exam 35 % Final 45 % Homeworks 20 % • Exams: No cheating, please. If do so, students may be punished by the school rules without any excuses. Syllabus • Homework: Will not be graded, but I encourage you to work them out for your quizzes. Solutions will be provided a few days after the quiz. • Attendance: No class roll will be taken. In case of class absence, you need to hand me a strong evidence that you have an emergency or are under unusual circumstances. No excuses will be accepted. Lecture plan Week Subject • 1 Introduction, Semiconductor materials • 2, 3 Schrodinger equation, Effective mass, Electrons and holes, Energy bands • 4,5 Bonds, Carrier Concentration, Crystallography • 6 Carrier Transport Phenomena ------ midterm ------ Lecture plan Week Subject • 7 Classification of semiconductors • 8-10 Semiconductor devices • 11 Dielectric materials, Refractive index • 12 Photonic Devices • 13 Basic Semiconductor Fabrication Introduction • Electronic industry has become the largest industry in the world since 1998. • Semiconductor devices are the foundation of this kind of industry. • In order to understand how electronic devices including optoelectronic devices work, we need to be familiar with material properties and electron behavior in the material. GWP -The semiconductor industry trending to grow at very high rate. - S/C industry have a contribution of 25% of the electronic industry in the early of 21st century. Basic block of S/C devices (a) (b) (c) (d) Metal-semiconductor interface; p-n junction; Heterojunction interface; Metal-oxide-semiconductor structure. Metal-semiconductor − A metal-semiconductor contact was the first semiconductor device in 1874. − This can be used as a rectifying contact* or as an ohmic contact*. − We can use this contact in many devices such as MESFET(metalsemiconductor field-effect transistor) where rectifying contact used as a gate and ohmic contacts as a source and a drain. *rectifying contact allows current to flow easily only in one direction. *ohmic contact passes current in either direction with a negligibly small voltage drop. p-n junction • Formed by putting p-type semiconductor (positively charged carriers) to n-type semiconductor (negatively charged carriers). • This is a key building block for most semiconductor devices. • By adding another p-type semiconductor, p-n-p bipolar transistor can be formed, but if three p-n junctions are used, this can form p-n-p-n device called a thyristor. Heterojunction interface • The heterojunction interface is formed between two different semiconductors. This kind of junction is the key component for high-speed and photonic devices. Metal-Oxide-Semiconductor structure • The metal-oxide semiconductor is famously called MOS structure. • This structure usually uses with two p-n junctions to form a famous device called MOSFET (MOS fieldeffect transistor). Major S/C devices Semiconductor materials • We may group solid-state materials by using electrical conductivities σ into 3 classes: insulators, semiconductors, and conductors. - Insulators have very low conductivities (10-18 – 10-8 S/cm) such as quartz or glass. - Conductors have high conductivities (104 – 106 S/cm) such as copper and silver. - S/C have conductivities between those of insulators and those of conductors. Semiconductor materials • The conductivity of a semiconductor is sensitive to temperature, illumination, magnetic field, and amounts of impurity atoms. • This sensitivity makes semiconductor one of the most important materials for electronic applications. Semiconductor materials Periodic Table Semiconductor materials • If we look at the periodic table, the element semiconductors, such as silicon (Si) or germanium (Ge), can be found in column IV of the table. • In the early 1950s, Ge was the most important semiconductor material, but, since the early 1960s, Si has played a major role and virtually displaced Ge as the main material for semiconductor material Semiconductor materials • The reasons of that are: − Better properties at room temperature − High-quality silicon dioxide (SiO2) can be grown thermally. − Si is second only to oxygen in great quantity. − Devices made from Si cost less than any other semiconductor material − Silicon technology is by far the most advanced among all semiconductor technologies. Electrons • Electrons behave like a wave and a particle at the same time. There is no theory or experiment to explain this electron’s behavior. • If we consider electron as a particle, we may start from the study of response of electrons to perturbation such as electric field, magnetic field, or EM waves. Resistivity and Mobility l A = cross section area V A voltage V is applied across a conductor of length “l” and cross section area “A”. Resistivity and Mobility From Ohm’s law: V I R l 1 l R A A where = resistivity [Ω-m] = conductivity [S/m] = 1/ Resistivity and Mobility I AV l I V A l E where V/l = E (electric field) J = current density [A/m2] J E Resistivity and Mobility Under influence of electric field, electron experience a force F eE ma eE a m where e = electron charge = 1.6 x 10-19 C m = mass of electron a = acceleration Resistivity and Mobility • Without any applied electric field, the random motion of electron leads to zero net displacement over a long period of time. • The average distance between collisions is called the mean free path. • The average time between collisions is called the mean free time, . • With applied electric field, electron does not have constant acceleration. It suffers collision that leads it to move with an average velocity called “drift velocity”. Resistivity and Mobility A drift velocity can be written as vD a e vD E m vD e E where µe = mobility of electron [m2/V-s] Resistivity and Mobility Resistivity and Mobility By moving electrons in conductor, this leads to have a current proportional to number of electrons crossing a unit area [m2] per unit time. J Ne .e.vD where Ne = number of free electrons per unit volume Resistivity and Mobility As electric field E increases, vD also increases, therefore, J also increases. This makes the conductor behave like a perfect source. However, the velocity vD saturates to a maximum value limited by thermal velocity. The mean thermal velocity (vthermal) of electron can be found from 1 2 3 mvthermal kT 2 2 Resistivity and Mobility 1 2 3 mvthermal kT 2 2 where m = effective mass of electron k = Boltzmann’s constant = 1.38 x 10-23 J/K T = absolute temperature (K) kT/2 = average thermal energy of electron in one-dimension J ( Neee ) E Neee e ( Nee) where Nee = charge density Resistivity and Mobility • The conductivity depends on the charge density and mobility. • Metals have high conductivity due to their high density of electrons although their mobilities (μm/t ~ 10 cm2/V-s)are very low compared to those of semiconductors (μS/C ~ 103 cm2/V-s). Resistivity and Mobility • The mobility is linearly dependent to the mean free time between collisions which is caused by two major mechanisms: lattice scattering and impurity scattering. • Lattice scattering is caused by the thermal vibrations of the lattice atoms at any temperature above absolute zero. As the temperature gets higher, the mobility will get lower. This shows that the mobility will decrease in proportion to T-3/2. Resistivity and Mobility • Impurity scattering is caused when a charge carrier past an ionized dopant impurity. • The carrier will be deflected due to the Coulomb force. The probability of impurity scattering depends on the total concentration of ionized impurities. • Unlike lattice scattering, for impurity scattering, the mobility due to impurity scattering will increase as the temperature gets higher. • This mobility in this case is shown to vary as T3/2/NT, where NT is the total impurity concentration. Resistivity and Mobility where µL = mobility due to lattice scattering µI = mobility due to impurity scattering 1 1 1 L I Resistivity and Mobility In semiconductors, both electrons and holes contribute to current in the same direction. Hole current and electron current are not necessarily equal because they have different effective masses. J S / C Neee N heh E S / C N e e N h h e Ex. Calculate the mean free time of an electron and mean free path having a mobility of 1000 cm2/V-s at 300 K. Assume me = 0.26m0, where m0 = electron rest mass = 9.1 x 10-31 kg. Ex. Calculate the mean free time of an electron and mean free path having a mobility of 1000 cm2/V-s at 300 K. Assume me = 0.26m0, where m0 = electron rest mass = 9.1 x 10-31 kg. Soln me e e (0.26 9.1 1031 kg)(1000 10 4 m 2 .V -1.s -1 ) 1.6 1019 C 0.148 ps From (5), mv 2 3kT 2 2 3kT vth 105 m/s m l vth 14.8 nm s v ; t Ex. In metals, μe = 5 x 10-3 m3/(V-s) and l = 1 cm, V = 10 volts is applied. Find the drift velocity vD and compare to thermal velocity vth. Ex. In metals, μe = 5 x 10-3 m2/(V-s) and l = 1 cm, V = 10 volts is applied. Find the drift velocity vD and compare to thermal velocity vth. Soln V vD e E e l 2 3 m 10 V 5 10 2 V.s 10 cm vD 5 m/s 3kT 3 1.38 1023 J/K 300K vth m 9.1 1031 kg vth 1.17 105 m/s vD vth The Hall Effect d Assume a p-type semiconductor sample, with electric field applied along x-direction and a magnetic field applied along z-axis, the Lorentz force qv x B (= qvxBz) due to the magnetic filed will exert an average upward force on the holes flowing in the x-direction. The Hall Effect d Therefore, drifting holes experienced an upward force which deflects holes upward toward the top of the sample and makes them accumulate there. This sets up an electric filed EH in y-direction called “Hall field”. This establishment of the electric field is known as the Hall Effect. The Hall Effect This establishment of the electric field is known as the Hall Effect. At the steady-state, the electric field along the y-axis exactly balances the Lorentz force (or it is called “an equilibrium”); that is qEy qvx Bz or Ey vx Bz The Hall Effect N h qEH B 1 EH BJ Nhq 1 where RH Hall coefficient Nhq J N h qv This Hall coefficient for n-type semiconductor is similar to the p-type one except it has an opposite sign as 1 1 RH qNe Ne e The Hall Effect This Hall effect is often used to distinguish an n-type from a p-type sample and also used to calculate the free charge density and the carrier mobility if the conductivity is known. For example, we know that the induced voltage VH known as “Hall Voltage” between the top and bottom is expressed by VH EH d The Hall Effect Using a voltmeter to measure VH then VH EH d I J Wd 1 EH B.J Nee VH 1 B.J d Nee BI Ne VH eW The Hall Effect If the conductivity σ is known, mobility can be found as ( Ne e) e e RH e e RH The Hall Effect Ex. A sample of Si is doped with 1016 phosphorus atoms/cm3. Find the Hall voltage in a sample with d = 500 μm, A = 2.5 x 10-3 cm2, I = 1 mA, and Bz = 1 Tesla. Note: 1 Tesla = 1 Wb/m2 = 104 G. The Hall Effect Soln 1 1 3 RH 625 cm /C 19 16 -3 eN e 1.6 10 C 10 cm VH EH d R .I .B RH .J .B d H d A cm3 103 A 104 Wb 4 625 500 10 cm -3 2 2 C 2.5 10 cm cm VH 1.25 mV