week5
advertisement
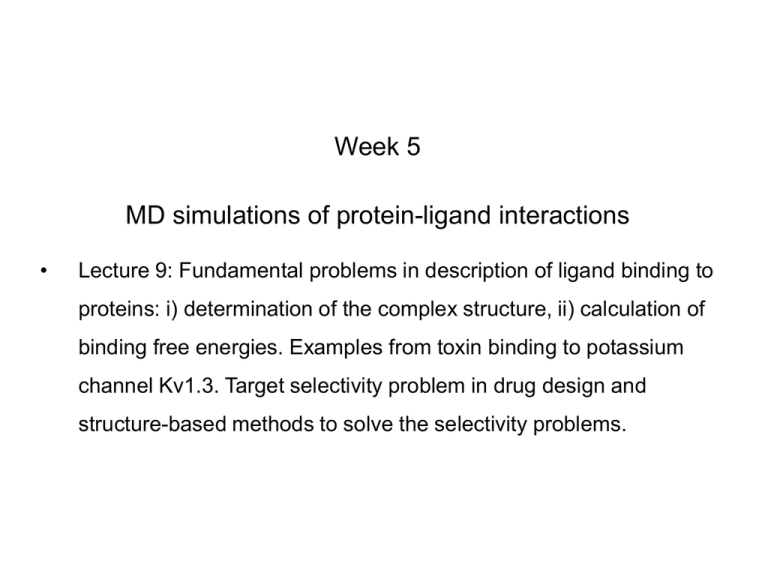
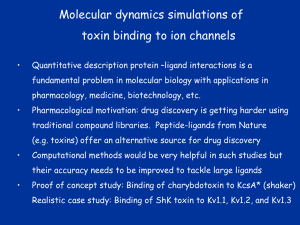
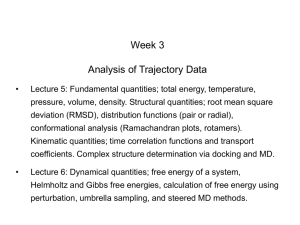
Week 5 MD simulations of protein-ligand interactions • Lecture 9: Fundamental problems in description of ligand binding to proteins: i) determination of the complex structure, ii) calculation of binding free energies. Examples from toxin binding to potassium channel Kv1.3. Target selectivity problem in drug design and structure-based methods to solve the selectivity problems. Why study proteinligand interactions? • Quantitative description of protein–ligand interactions is a fundamental problem in molecular biology • Pharmacological motivation: drug discovery is getting harder searching compound libraries using experimental methods. Using computational methods and peptide ligands from Nature (e.g. toxins) offer alternative methods and means for drug discovery • Computational methods would be very helpful in drug design but their accuracy needs to be confirmed for larger, charged peptide ligands • Proof of concept study: Binding of charybdotoxin to KcsA* (Shaker) Realistic case study: Binding of ShK toxin and analogues to Kv1.1, Kv1.2, and Kv1.3 channels Two essential criteria for development of drug leads 1. Should bind to a given target protein with high affinity 2. Be selective for the target protein The first issue is addressed with many experimental (e.g. HTS) and computational methods (e.g. docking), and there is a huge data base about high affinity ligands. The second issue is harder to address with traditional methods and would especially benefit from a rational drug design approach. Example: Kv1.3 is one of the the main targets for autoimmune diseases • ShK toxin binds to Kv1.3 with pM affinity • But it also binds to Kv1.1 with pM affinity • Need to improve selectivity of ShK for Kv1.3 over Kv1.1 Challenges in computational design of drugs from peptides 1. Apart from a few cases, the complex structure is not known. Assuming that structures (or homology models) of protein and ligand are known, the complex structure can be determined via docking followed by refinement with MD simulations. 2. Affinity and selectivity of a set of ligands for target proteins need to be determined with chemical accuracy (1 kcal/mol). Binding free energies can be calculated accurately from umbrella sampling MD simulations. For selectivity, one could use the free energy perturbation (FEP) method (computationally cheaper). The FEP method is especially useful if one is trying to improve selectivity via minor modifications/mutations of a ligand. Computational program for rational drug design from peptides 1. Complex structure determination: Find the initial configuration for the bound complex using a docking algorithm (e.g., HADDOCK). Refine the initial complex(es) via MD simulations. 2. Validation: a) Determine the key contact residues involved in the binding and compare with mutagenesis data to validate the complex model. b) Calculate the potential of mean force for the ligand, determine the binding constant and free energy, and compare with experiments. 3. Design: Consider mutations of the key residues on the ligand and calculate their binding energies (relative to the wild type) from free energy perturbation in MD simulations. Those with higher affinity/selectivity are candidates for new drugs. Proof of concept study: Binding of charybdotoxin (ChTx) to KcsA* (shaker mimic) • Complex structure is determined from NMR, so it provides a unique test case for MD simulations of peptide binding. • Using HADDOCK for docking followed by refinement via MD simulations reproduces the experimental complex structure. • Binding free energy of ChTx calculated from the potential of mean force (PMF): -7.6 kcal/mol • experimental value: -8.3 kcal/mol Structure of the KcsA*- ChTx complex Important pairs: K27 - Y78 (ABCD) R34 - D80 (D) R25 - D64, D80 (C) K11 - D64 (B) K27 is the pore inserting lysine – a common thread in scorpion and other K+ channel toxins. K11 R34 Realistic case study: ShK toxin binding to Kv1 channels • Motivation: – Kv1.3 is the main target for autoimmune diseases – ShK binds to Kv1.3 with pM affinity (but also to Kv1.1) – – Need to improve selectivity of ShK for Kv1.3 over Kv1.1 Some 400 ShK analogues have been developed for this purpose 1. Find the complex structures of ShK with Kv1.1, Kv1.2 and Kv1.3, and validate them using mutagenesis data. Determine the PMFs and the binding free energy and compare with experiment. 2. Repeat the above study for ShK-K-amide (an analogue with improved selectivity) to rationalize the experimental results. 3. WT complex structures indicate that K18A mutation should improve selectivity. Perform PMF and FEP calculations to quantify this prediction. NMR structure of ShK toxin ShK toxin has three disulfide bonds and three other bonds: D5 – K30 K18 – R24 T6 – F27 These bonds confer ShK toxin an extraordinary stability not seen in other toxins Homology model of Kv1.3 Can be obtained from the crystal structure of Kv1.2 (over 90% homology and 1-1 correspondence between residues). Note: care must be exercised for the V H404 mutation because H404-D402 side chains cross link (several publications have the wrong Kv1.3 structure because of this). Kv1.1-ShK complex Monomers A and C Monomers B and D Kv1.3-ShK complex Monomers A and C Monomers B and D Pair distances in the Kv1.3-ShK complex (in A) Kv1.3 ShK Dock. MD av. Exp. D376–O1(C) R1–N1 5.0 4.5 S378–O(B) H19–N 3.2 3.0 ** Y400–O(ABD) K22–N1 2.9 2.7 ** G401–O(B) S20–OH 2.9 2.7 ** G401–O(A) Y23–OH 3.5 3.5 ** D402–O(A) R11–N2 3.2 3.5 * H404-C(C) F27-Ce1 9.7 3.6 * V406–C1(B) M21–Ce 9.4 4.7 * D376–O1(C) R29–N1 12.2 10.2 * HADDOCK is not very good for hydrophobic int’s ** strong, * intermediate ints. (from alanine scanning Raucher, 1998) R24 (**) and T13 and L25 (*) are not seen in the complex (allosteric) Average pair distance as a function of umbrella window positions ** denotes strong coupling and * intermediate coupling * * ** ** * ** ** RMSD of ShK as a function of umbrella window The RMSD of ShK relative to the NMR structure remains flat throughout Overlap of the neighbouring windows Gaussian dist: % overlap 1 erf (d / 8 ), d : distance, kBT / k For k=30 kcal/mol/A2, the overlap is about 10% in bulk, which is an overlap optimal value for umbrella simulations (only one extra window needed) Convergence of the PMF for the Kv1.3-ShK complex PMF of ShK for Kv1.1, Kv1.2, and Kv1.3 Comparison of the binding free energies of ShK and its analogues to Kv1.x channels Complex Gb(PMF) Gb(exp) (kcal/mol) Kv1.1–ShK -14.3 ± 0.6 -14.7 ± 0.1 Kv1.2–ShK -10.1 ± 0.6 -11.0 ± 0.1 Kv1.3–ShK -14.2 ± 0.7 -14.9 ± 0.1 Kv1.1-ShK-K-amide -11.8 ± 1.0 -12.3 ± 0.1 Kv1.3-ShK-K-amide -14.0 ± 0.4 -14.4 ± 0.1 Kv1.1-ShK[K18A] -11.7 ± 0.7 -11.3 ± 0.1 Kv1.3-ShK[K18A] -13.9 ± 0.6 -14.2 ± 0.1 Excellent agreement with experimental values for all channels, which provides an independent test for the accuracy of the complex models. ShK[K18A] analogue should increase Kv1.3/Kv1.1 selectivity • All the single and some double mutations in ShK have been patented by a pharmacology company (AMGEN), which indicated that none are useful for design of a selective analogue. • As a result, these mutations have not been considered in addressing the selectivity problem. Instead people have been looking for nonnatural analogues, which have other problems. • The Kv1-ShK complex structures indicate several mutations that should improve Kv1.3/Kv1.1 selectivity (e.g. K18A, R29A) • The K18A mutation does not change the binding mode in either Kv1.1ShK or Kv1.3-ShK complex (while R29A does). Thus first consider the K18A analogue • Test case: use both the FEP/TI and PMF calculations to predict the free energy change due to the K18A mutation. Kv1.1 & Kv1.3 complexes with ShK[K18A] (ShK orange) Kv1.3 complex with ShK (transparent) and ShK[K18A] Free energy perturbation calculations for ShK[K18A] • The K18A mutation does not change the binding mode in either Kv1.1-ShK or Kv1.3-ShK complex. Thus one can use FEP calculations to find the free energy change due to the mutation. • Straightforward FEP calculation of the K18A mutation does not work. • Split the Coulomb and Lennard-Jones parts and do a staged FEP calculation • In the binding site, K K0 A0 A, while in the bulk follow the opposite cycle, i.e., A A0 K0 K • Add the three contributions from K K0 , K0 A0 and A0 A steps to find the binding free energy difference, DDG(K A) Thermodynamic cycle for the FEP/TI calculations DDGb DGb (ShK[K18A] ) DGb (ShK) PMF DDG (K K 0 ) DDG (K 0 A 0 ) DDG (A 0 A) FEP/TI Effect of the K18A mutation on binding free energies Binding free energy differences for Kv1.1 and Kv1.3, and the selectivity free energy for Kv1.3/Kv1.1. (in units of kcal/mol) ∆∆Gb(Kv1.1) ∆∆Gb(Kv1.3) ∆∆Gsel FEP 2.1 0.5 1.6 TI 2.4 0.2 2.2 PMF 2.7 0.4 2.3 Exp. 3.1 0.8 2.3 DDGb DGb (ShK[K18A] ) DGb (ShK ) DDGsel DDGb (Kv1.1) DGb (Kv1.3) Summary • Reliable protein-ligand complex structures can be obtained using docking methods followed by refinement via MD simulations. (Complex models have been validated via mutagenesis data) . • Binding free energies can be determined near chemical accuracy (i.e., 1 kcal/mol) from PMF. • Once a protein-ligand complex is characterized, one can study the effects of mutations on the ligand by performing FEP calculations, provided that the binding mode is preserved. These will be especially useful when seeking mutations that will increase affinity or improve selectivity of a given ligand targeting a specific protein.