Mossbauer
advertisement
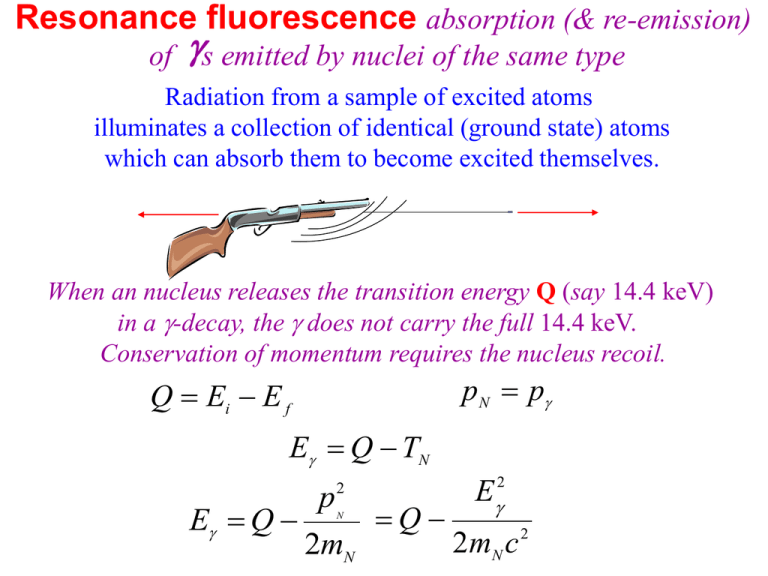
Resonance fluorescence absorption (& re-emission) of s emitted by nuclei of the same type Radiation from a sample of excited atoms illuminates a collection of identical (ground state) atoms which can absorb them to become excited themselves. When an nucleus releases the transition energy Q (say 14.4 keV) in a -decay, the does not carry the full 14.4 keV. Conservation of momentum requires the nucleus recoil. pN p Q Ei E f E Q TN 2 E2 p Q E Q 2mN c 2 2mN N If this change is large enough, the will not be absorbed by an identical nucleus. E emitted Q p2 2mN c 2 In fact, for absorption, actually need to exceed the step between energy levels by enough to provide the nucleus with the needed recoil: 2 2 p p N TN = = 2mN 2mN p=E /c E absorbed Q E2 2mN c 2 The photon energy is mismatched by 2 E2 2mN c 2 E2 mN c 2 For atomic resonance experiments, note Q ~ few eV (for visible transitions) and mN ~ Au ~ A1000 MeV (1 10eV ) TN 1012 – 1010eV (10 100)1000 MeV 2 But how precisely fixed is the emitted energy anyway? Recall: there is a “natural width” to the energy, related to how stable the initial energy state was. For atomic transitions, the typical lifetime is ~108 sec The energy uncertainty E 10 eV 7 Notice the uncertainty E >> 2TN 2TN Q with an enormous amount of overlap allowing resonance fluorescence For NUCLEAR resonance experiments Q ~ few MeV (for -ray emissions) with mN ~ Au ~ A1000 MeV (100keV 10MeV ) TN 0.1 – 104eV (10 100)1000 MeV 2 For nuclear transitions, the typical lifetime is ~1010 sec The energy uncertainty E 10 eV 5 This time the uncertainty E << 2TN 2TN Q which provides no overlap allowing resonance fluorescence As an example consider the distinctive 14.4 keV from 57Fe. ~90% of the 57Fe* decays are through this intermediate level produce 14.4 keV s. =270d 57Co 7/2 The recoil energy of the iron-57 nucleus is EC 5/2 136keV Erecoil =10-7s 57Fe 3/2 14.4keV 1/2 E2 2 mN c 2 (14.4keV ) 2 0.002eV 2(53.022GeV ) With = 107 s, =108 eV this is 5 orders of magnitude greater than the natural linewidth of the iron transition which produced the photon! 1958 Rudolf Mössbauer Working with 129-keV ray of 191Ir Discovered by imbedding the radioactive samples in crystals, and cooling them, their tightly held crystal positions prevented them from recoiling. The energy of recoil had been absorbed by the lattice as a whole. To keep the within its natural linewidth how many iron nuclei would have to recoil together in our example of 57Fe? 2 Erecoil (14.4keV ) 10 eV 2( N 53.022GeV ) 8 N 200,000 Very small compared to Avogadro's number! (In fact a speck too small to be seen in a microscope). Any tiny crystal within a 57Cobalt-containing piece of iron would meet the conditions for resonance absorption if cooled sufficiently. You can also destroy that resonance by moving the source relative to the absorber and Doppler shifting the photons off resonance. The Doppler shift of a photon is a relativistic shift given by observed source 1 v / c 1 v / c v is positive for an approaching source This can be written as vo vs (1 v / c)(1 v / c) vs (1 v / c) 2 2 (1 v / c)(1 v / c) (1 v c ) If v/c <<1 this simplifies to vo vs (1 v / c) shift recoiling emissions to resonance by moving the source relative to the absorber and Doppler shifting the photons to the necessary energy for absorption. Continuing our 57Fe example: The source velocity necessary to shift the photon to resonance absorption energy is v v 0.002eV h ( v0 vs ) hvs 14.4keV c c v 42 m/sec This was in fact demonstrated with the source in a centrifuge Cool an embedded sample to produce recoilless emission and drive the source or absorber to scan the resonance. radioactive source absorbing sample detector vibrator servo-motor controls data acquisition Continuing with our example of 57Fe : Using the uncertainty in energy given by as a measure of how far you need to Doppler shift frequencies to be off resonance: Setting 108 eV 14.4keV v / c gives: 0.0002 meter/sec = 2 mm/sec Mossbauer Absorption of 191Ir 129-keV gamma rays from iridium-191 were measured as a function of source velocity. A velocity of only about 1.5 cm/s was enough to drop the absorption to half its peak value. Sample and absorber were cooled to 88K. 191Ir v 191Ir Detector Source Absorber A half-width of only about 0.65 x 10-5 electron volts makes this absorption an extremely sensitive test of any influence which would shift the frequency. It is sensitive enough to measure the Zeeman splittings from the magnetic field of the nucleus. The incredibly high resolution of the Mössbauer effect in 57Fe makes possible the measurement of the nuclear Zeeman effect . O. C. Kistmer and A. W. Sunyar, Physical Review Letters, 4, 412(1960). The splittings are 11 orders of magnitude smaller than the nuclear transition energy! Nuclear Hyperfine Interactions Observable with Mossbauer Spectroscopy Observed Effect Isomer Shift Interaction of the nuclear charge distribution with the electron cloud surrounding the nuclei in both the absorber and source Zeeman Effect(Dipole Interaction) Interaction of the nuclear magnetic dipole moment with the external applied magnetic field on the nucleus. Quadrupole Splitting Interaction of the nuclear electric quadrupole moment with the EFG and the nucleus Illustration Observed Spectrum http://www.fastcomtec.com/fwww/moss.htm http://www.cryoindustries.com/moss.htm http://www.dwiarda.com/scientific/Moessbauer.html Gravitational redshift A ``gedänken'' experiment first suggested by Einstein: A particle of rest mass m is dropped to fall freely with an acceleration g from a tower of height h. It reaches the ground with a velocity v 2 gh , so its total energy E, as measured by an observer at the foot of the tower is 1 2 4 Ebottom mc mv O ( ) 2 2 4 mc mgh O ( ). 2 Let the particle rebound elastically at the bottom and return. Etop mc 2 Ebottom mc mgh O ( ). 2 4 Suppose the rebounding particle could be converted to a photon of energy Ebottom & upon its arrival at the top changed back into a particle of rest mass m = E/c2. Should mtop=mbottom? Or is the mass now greater than it began? What must be true, even for the photon is Etop Ebottom 2 mc 2 . 4 mc mgh O ( ) Etop Ebottom mc 2 4 mc mgh O ( ) Etop Ebottom 1 2 4 2 1 gh / c O ( ) / mc 2 Etop Ebottom gh 1 2 . c Since for photons we have Etop = htop top vbottom1 gh / c 2 This implies a photon climbing in the earths gravitational field will lose energy and consequently be redshifted. vbottom top vbottom vbottom1 gh / c vbottom[1 1 gh / c ] 2 vbottomgh / c 2 2 The redshift is: vbottom top 2 gh / c vbottom In just 22.6 meters, the fractional redshift 0 1 gh / c 2 is only 4.92 10-15 but using the Mössbauer effect on the 14.4 keV gamma ray from 57Fe should provide high enough resolution to detect that difference! In the early 60's physicists Pound, Rebka,and Snyder at the Jefferson Physical Laboratory at Harvard measured the shift to within 1% of this predicted shift. Pound, R. V. and Rebka, G. A. Jr. "Gravitational Red-Shift in Nuclear Resonance." Phys. Rev. Lett. 3, 439-441, 1959. The gain in energy for a photon which falls distance h = 22.6 m is E 14.4keV E mgh 2 gh g 22.6m 2 c c E 3.5 10 eV 11 Comparing the energy shifts on the upward and downward paths gives a predicted difference 2(3.5 10 eV ) E E 15 4.9 10 14.4keV E down E up 11 The measured difference was E E 15 (5.1 0.5) 10 E down E up