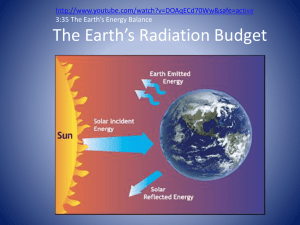
Radio waves belong to a family The
advertisement

Radio Waves Where do they come from? Radio waves belong to a family • The electromagnetic spectrum (EM) is a continuum of waves, sometimes called electromagnetic radiation. • These waves may be created in a number of ways, but all share the following characteristics: Family Trait 1: No Medium • EM waves do not require a medium to move from source to observer. • Mechanical waves (such as sound) must travel through a medium – “In space, no one can hear you scream” • Tagline from the movie Aliens. This is one (rare) example of Hollywood getting the science right! Family Trait 2: Same Speed • They all travel at the same speed, c, the speed of light in a vacuum – an unfortunate choice, since most people don’t associate light and radio as being part of the same EM spectrum – c ≈ 3 x 108 m/s • When traveling through a medium, the wave speed, v, is found by the formula v = c/n – where n is the index of refraction of the medium through which the wave is moving Family Trait 3: Fixed relationship • Frequency (f) and wavelength (λ) are related as f = c/λ • This is an inverse relationship: the larger (higher) the frequency gets, the smaller the wavelength becomes • Note to teachers: Throughout our presentations, we will be using f for frequency. The Greek letter nu (ν) is traditionally used for frequency, unfortunately it looks too much like a vee (v) in most fonts. The inconsistency in notation is a constant source of confusion for introductory physics students! Family Trait 4: Wave behavior • Physical Properties – – – – Wavelength Frequency Amplitude Phase • Behaviors – – – – – Reflection Refraction Diffraction Interference Doppler Shift Family Trait 5: Particle Behavior • The frequency of an EM wave is related to its energy by the formula f = E/h (more commonly written as E = h·f) h is Planck’s constant = 6.626 x 10-26 J/Hz • This behavior is attributed to a particle called a photon. That EM radiation appears to be both a wave and a particle is called “wave-particle duality” (to be discussed in another section) • The wave behavior dominates the lower frequency spectrum (Radio waves), while the particle nature shows itself more readily in the higher frequencies EM Spectral Bands • For convenience, scientists have divided the spectrum into bands. Those bands are, in order of increasing wavelength (decreasing frequency): • Gamma Rays, X-Rays, Ultraviolet, Visible, Infrared, Microwave, and Radio – Microwaves are often considered part of the Radio band Images/animations courtesy NRAO / AUI / NSF Shorter Wavelengths Longer Wavelengths Anatomy of EM waves • EM waves consists of a traveling electric field (E) and a traveling magnetic field (B). The E and B fields are in-phase and orthogonal (at right angles) to one another. Image/animation courtesy NRAO / AUI / NSF Human detections of EM waves • Humans have built-in detectors of EM waves, called eyes. We see EM waves in the Visible part of the Spectrum. • Sound is NOT part of the EM spectrum!!! – Sound is a mechanical wave, which requires a medium. Humans have a different set of detectors for mechanical waves, called ears. Natural Radio Sources • Lightning, sparks • Solar System – our sun, planets • Milky way – star forming regions, old stars, supernova remnants, Galactic center • Extragalactic – quasars, radio jets • Molecules What causes Radio waves? • Vibrating atoms and molecules – Thermal vibrations due to temperature – Rotational energy for asymmetric molecules • Excited atoms and molecules – Absorption/emission of energy (a photon) • Accelerating charged particles – Movement in electric or magnetic fields Two categories of radio sources • Broadband – Spectral content of source is spread out across many of the EM bands (radio, visible, x-ray) – Observations made in the radio band should correlate with other parts of the EM spectrum (see Sun) • Narrowband – Attributes of the source favor one part of the spectrum (or single frequencies) over others – For example: you can’t see the Ozone in the Mesosphere in the visible spectrum, but we can detect their radio waves Broadband Radiation • Broadband radio signals usually have a thermal origin. – Blackbody radiation (Planck’s Law) • All objects radiate EM waves in proportion to their internal temperature – Thermal Bremsstrahlung • Acceleration of a charged particle (electron) by another charged particle (nucleus) • Includes cyclotron and synchrotron radiation Blackbody Radiation • Any object above absolute zero will emit a broad spectrum of radiation • The peak of the curve shifts to shorter wavelengths as temperature increases Image courtesy NRAO / AUI / NSF Thermal Bremsstrahlung • Also called free-free radiation • Electrons whizzing by ions Image/animation courtesy NRAO / AUI / NSF Non-Thermal Radiation • Most common: Synchrotron radiation • Usually electrons accelerating in a magnetic field Image/animation courtesy NRAO / AUI / NSF Another Non-Thermal Source • MASERs (Microwave Amplification by Stimulated Emission of Radiation) – like a LASER, only at radio frequencies, not visible to the eye • Usually associated with molecules in stellar gas clouds Image/animation courtesy NRAO / AUI / NSF The Sun in different “light” Radio Visible Ultraviolet X-Ray Images courtesy of: NRAO/AUI/NSF/G. Dulk, D. Gary (radio), NSO/AURA/NSF (visible) SOHO/NASA (ultraviolet) and Yohkoh/ISIS/NASA (X-Ray) Narrowband Radiation • Electron energy transitions tend to emit visible or UV radiation • Vibrational transitions tend to emit IR radiation (mm waves) • Rotational transitions tend to emit microwave radiation (Radio waves) – The molecule must have an electric dipole moment Example: 21 cm Hydrogen Line Image/animation courtesy NRAO / AUI / NSF Molecules found in space Simple Hydrides, Oxides, Sulfides, Haloids H2 CO NH3 CS NaCl HCl SiO SiH4 SiS AlCl H2O SO2 C2 H2S KCl OCS CH4 PN AlF Nitriles, Acetylenes and Derivatives C3 HCN CH3CN HNC C2H4 C5 HC3N CH3C3N HNCO C2H2 C3O HC5N CH3C5N HNCS C5O HC7N CH3C4H HNCCC C3S HC9N CH3C4H CH3NC C4Si HC11N C2H5CN HCCNC Aldehydes, Alcohols, Aethers, Ketones and Amides H2CO CH3OH HCOOH CH2NH CH2C2 H2CS C2H5OH CH3COOH CH3NH2 CH2C3 CH3CHO CH3SH (CH3)2O NH2CN NH2CHO (CH3)2CO H2CCO Cyclic Molecules C3H2 SiC2 C-C3H Molecular Ions CH HCO+ HCNH+ H3O+ HN2- HCS+ HOCO+ SO+ HOC+ H2DRadicals OH C3H CN C2O C2S CH C4H C3N NO NS C2H C5H HCCN SO SiC CH2 C6H CH2CN HCO SiN NH MgNC CP 11.0724545 GHz Ozone Line • The Mesospheric Ozone line we detect with the MOSAIC system is a change in rotation of the asymmetric ozone molecule • This is a quantum mechanical effect, due to the existence of discrete energy levels of rotational angular momentum Atmospheric opacity • As the illustration below shows, there are many EM frequencies which do not pass through our atmosphere, due to absorption by atoms and molecules present. Images courtesy of NASA