Lecture 13 Power point notes
advertisement

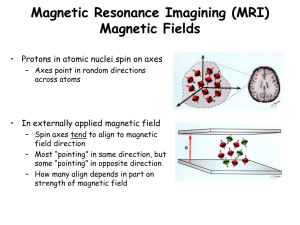
Nuclear Magnetic Resonance
• Absorption of electromagnetic radiation from 4
MHz to 900 MHz
• Nuclear process
Radiation absorbed by nuclei
• Sample must be placed in strong magnetic field
• Used for determining structure
• Two types of NMR
Continuous wave
Pulsed wave
Most spectrometers are pulsed wave (FT)
Theory
Environmental effects on spectra
Spectrometers
Applications
11-1
Theory
• Quantum description
Spin
Nuclei with spin have angular momentum (p)
p is integral or half integral multiple of h/2p
For a given p, maximum spin values is I
* Spin quantum number
Nuclei has 2I+1 states
* m=I, I-1, ….., -I
States energies differ in magnetic field
• For proton
p=1/2
m= ½, -1/2
11-2
Energy levels
• Magnetic moment
becomes orientated in
two directions
½ is lower E, -1/2 is
higher
h
+ or - E
B
4p
B
0
from E h 0
2p
11-3
Distribution
• What is distribution between states
Based on Boltzmann’s equation
N 1/ 2
hB
exp(
)
N1/ 2
2pkT
• For proton in 4.69 T field at 20 °C
N 1/ 2
2.68e8 * 6.63E 34* 4.69
exp(
) 0.999967
N1/ 2
2p 1.38E 23* 293
N1/ 2
1.000033
N 1/ 2
11-4
Nuclei properties
isotope
spin I
natural
abundance
[%]
gyromagnetic
ratio, (gamma)
[107*rad/(T*s)]
relative
sensitivity
absolute
sensitivity
1H
1/2
99.98
26.7519
1.00
1.00
2H
1
0.016
4.1066
9.65 · 10-6
1.45 · 10-6
12C
0
98.9
--
--
--
13C
1/2
1.108
6.7283
1.59 · 10-2
1.76 · 10-4
14N
1
99.63
1.9338
1.01 · 10-3
1.01 · 10-3
15N
1/2
0.37
-2.712
1.04 · 10-3
3.85 · 10-6
16O
0
98.9
--
--
--
17O
5/2
0.037
-3.6279
2.91 · 10-2
1.08 · 10-5
31P
1/2
100
10.841
6.63 · 10-2
11-5
-2
6.63 · 10
Theory
• Magnetic moment related to magnetogyric
ratio ()
mp
11-6
Procession in a magnetic field
• Angular velocity (radians/s)
wB
• Larmor frequency
B
2p
11-7
Relaxation Process
• Non-radiative relaxation processes (thermodynamics!).
If the relaxation rate is fast, then saturation is
reduced
If the relaxation rate is too fast, line-broadening in
the resultant NMR spectrum is observed
• Two major relaxation processes;
Spin - lattice (longitudinal) relaxation
T1 relaxation time
Spin - spin (transverse) relaxation
11-8
Spin Lattice Relaxation
•
•
Nuclei in the lattice are in vibrational and rotational motion, which creates a
complex magnetic field
magnetic field caused by motion of nuclei within the lattice is called the
lattice field
lattice field has many components
Some components will be equal in frequency and phase to the
Larmor frequency of the nuclei of interest
These components of the lattice field can interact with nuclei in the
higher energy state
cause them to lose energy (returning to the lower state)
energy that a nucleus loses increases the amount of vibration and
rotation within the lattice (resulting in a tiny rise in the
temperature of the sample).
relaxation time, T1 (the average lifetime of nuclei in the higher energy state) is
dependant on the magnetogyric ratio of the nucleus and the mobility of the
lattice
As mobility increases, the vibrational and rotational frequencies
increase, making it more likely for a component of the lattice field to be
able to interact with excited nuclei
at extremely high mobilities, the probability of a component of the
lattice field being able to interact with excited nuclei decreases. 11-9
Spin-Lattice Relaxtion
11-10
Spin Lattice Relaxation
11-11
Spin-Lattice Relaxation
11-12
Name
Inversion Recovery
(IRFT)
Progressive Saturation
(PSFT)
Saturating Comb
(Mainly useful in
solid)
require: T2*<<T1
Pulse Sequence
D1-180-tau-90-Acq
{D1+Acq>5*T1}
signal evolution vs T1
M(tau)/M0= 1-2*exp(tau/T1)
(preceded by dummy pulses) - D1-90-Acq
{tau=D1+Acq}
M(tau)/M0= 1-exp(tau/T1)
{n*90-t}-tau-90-Acq
t: pulse spacing during Comb. :T2*< t <T1
tau: delay for magnetization recovery
M(tau)/M0= 1-exp(tau/T1)
11-13
Spin-Spin Relaxation
• Spin - spin relaxation describes the interaction between
neighboring nuclei with identical precessional
frequencies
differing magnetic quantum states
• nuclei can exchange quantum states
a nucleus in the lower energy level will be excited
the excited nucleus relaxes to the lower energy
state
no net change in the populations of the energy
states
the average lifetime of a nucleus in the excited
state will decrease
• can result in line-broadening
• T2
11-14
Spin-Spin Relaxation
11-15
Chemical Shift
Nuclear Shielding
•
•
•
•
•
Nuclei are shielded by
electrons.
Induced field associated
with orbiting electrons.
Require stronger magnetic
field than H0.
Increased shielding requires
greater applied field strength
to achieve resonance.
Chemical Shift (, ppm) =
•
•
•
•
A molecule may contain multiple
protons that exist in unique electronic
environments.
Therefore not all protons are shielded
to the same extent.
Resonance differences in protons are
very small (ppm).
Measure differences in resonance
energy relative to a reference.
Tetramethylsilane (CH3)4Si (TMS)
provides highly shielded reference
(set to 0ppm).
Observed chemical shift from TMS (Hz)
Sptectrometer frequencey (MHz)
= ppm
11-16
NMR Spectra
Representative peak,
3 equivalent protons
TMS
A
bs
or
ba
nc
e
H
C H
H
12
11 10
9
8
7
6
5
4
3
2
1
0 , ppm
Increasing magnetic field strength
Increased sheilding of nuclei
TMS as reference
is set to 0 ppm
• Hypothetical NMR spectra.
• Shows TMS reference.
• Chemical shifts (, ppm) given relative to TMS
11-17
Chemically Equivalent
H
H
• Protons in the same
environment will have the
same chemical shift.
• Protons in different
environments have different
chemical shifts.
• Protons with the same
chemical shift are referred to
as chemically equivalent.
• Integrated area of peak is
proportional to the number of
protons.
H
C
H
H
C
H
H
H
H
H
H
H
H
H
H
C
H
H
C
H
H
C
H
H
H
C
H
H
H
H
H
H
11-18
Sample Spectra
H
H
C
H
H
C
H
TMS
A
bs
or
ba
nc
e
H
12
11 10
9
8
7
H
6
5
4
3
2
1
0 , ppm
H
H
H
C H
TMS
H
H
H
A
bs
or
ba
nc
e
• The first spectra is that of a
symmetric molecule, all
protons are equivalent.
• Second spectra is that of a
molecule containing two
types of protons.
• Correlation chart for
proton chemical shift
12
11 10
9
8
7
6
5
4
3
2
1
0 , ppm
11-19
Type of Proton
Structure
Chemical Shift,
ppm
Cyclopropane
C3H6
0.2
Primary
R-CH3
0.9
Secondary
R2-CH2
1.3
Tertiary
R3-C-H
1.5
Vinylic
C=C-H
4.6-5.9
Carbonyl
Compounds
H-C-C=O
2-2.7
Acetylenic
triple bond,CCH
2-3
Aldehydic
R-(H-)C=O
9-10
Aromatic
Ar-H
6-8.5
Hydroxylic
R-C-OH
1-5.5
Benzylic
Ar-C-H
2.2-3
Phenolic
Ar-OH
4-12
Allylic
C=C-CH3
1.7
Enolic
C=C-OH
15-17
Fluorides
H-C-F
4-4.5
Carboxylic
RCOOH
10.5-12
Chlorides
H-C-Cl
3-4
Amino
RNH2
1-5
Bromides
H-C-Br
2.5-4
Iodides
H-C-I
2-4
Alcohols
H-C-OH
3.4-4
Ethers
H-C-OR
3.3-4
Esters
RCOO-C-H
3.7-4.1
Esters
H-C-COOR
2-2.2
Acids
H-C-COOH
2-2.6
11-20
11-21
11-22
Nuclear Shielding/Deshielding
(ppm)
CH3OCH3
(CH3)3N
4.3
3.2
2.2
CH2Cl2
CH3Cl
5.4
3.1
CHCl3
CH3CH3
0.9
E
le
c
tr
o
n
e
g
a
ti
ve
• Valence electron density can
shield nucleus from applied
field.
• Electronegative substituents
can draw elecron density
away.
• Results in deshielding.
• Anisotropy: p-electrons and
induced magnetic field.
• Results in shielding and
deshielding zones.
CH3F
7.3
S
u
b
st
it
u
e
n
ts
(ppm)
H
A
n
is
o
tr
o
p
y
H0
O
H
H
11-23
Spin-spin Splitting and n+1 Rule
•
•
•
•
•
•
n = 0, singlet
Each type of proton “senses”
protons on adjacent carbon atoms.
Spin state of nearby protons
contributes to the proton evironment
and apparent magnetic field.
General rule is that the signal is split
into n+1 peaks. n = number of
equivalent neighboring protons.
Spacing between component peaks
referred to as coupling constant (J).
J coupling is representative of the
degree to which protons interact.
J usually 0-18Hz
Splitting
+1/2
-1/2
+1
n = 1, doublet
0
-1
n = 2, triplet
-1 1/2
+1 1/2
+1/2
Numbers in Italics refer to net spin
-1/2
n = 3, quartet
Two types of protons in 1,1,2-trichloroethane
Cl
Ha
Hb
C
C
Cl
Hb
Cl
Cl
Ha
Hb
C
C
Cl
Cl
Hb
equivalent
protons behave
as a group
11-24
Splitting patterns
n = 0, singlet
Splitting
1
Pascal's Triangle
1
1
1
+1/2
-1/2
n = 1, doublet
+1
0
n = 2, triplet
3
3
1
4
6
1
-1
-1 1/2
+1 1/2
+1/2
Numbers in Italics refer to net spin
1
2
4
1
1
1
5
10
10
5
1
-1/2
n = 3, quartet
11-25
1,1,2-trichloroethane
Cl
Hb
J
Ha
Hb
C
C
Cl
Hb
Cl
TMS
Ha
A
bs
or
ba
nc
e
JJ
8
•
•
•
•
•
7
6
5
4
3
2
1
0 , ppm
NMR spectrum for 1,1,2-trichloroethane
Hb proton signal split into doublet
Ha proton signal split into triplet
J couplings are the same for Ha and Hb signals
Ha integral is 1/2 that of Hb
11-26
Magnetic Equivalence vs. Chemical
Equivalence
Br
Ha
• NMR differentiates between
nuclei based on environment.
• In constrained systems, two
protons on the same C-atom
can be in different
environments.
• These protons can
demonstrate spin-spin
splitting.
Br
H3C
Hb
H3C
Ha
Hc
Hb
X
11-27
Higher Field Strengths
TMS
A
bs
o
rb
an
ce
60 MHz
8
7
6
5
4
3
2
0 , ppm
1
60 MHz
TMS
100 MHz
A
bs
o
rb
an
ce
• At higher field strengths
differences in energy between
spin states is increases.
• Improved signal resolution.
• Coupling constants are
independent of field strength.
4
Chemical Shift (, ppm) =
3
2
1
100 MHz
0 , ppm
Observed chemical shift from TMS (Hz)
= ppm
Sptectrometer frequencey (MHz)
11-28
Carbon-13 NMR
•
•
•
•
•
•
~1.08% of C atoms are the 13C
isotope.
Do not usually see C-C spin-spin
interactions.
Can see coupling between C and
attached H’s.
Magnetic moment m of 13C is low.
Resonances of 13C nuclei are ~6000
fold weaker than 1H resonances.
Therefore most useful information
is chemical shift.
Covers a range of 0-200ppm.
Ethyl phenylacetate
O
H2C
Undecoupled
O
H2
C
CH3
Decoupled from protons
A
b
so
rb
a
n
ce
•
2,6
TMS
3,5
1
6
5
O
4
C
180
O
H2
C C
2
4
3
O CH2
CH3
1
160
140
120
100
80
60
40
20
11-29
0
, ppm
Proton Decoupled
O
H2C
Undecoupled
O
H2
C
CH3
Decoupled from protons
A
b
so
rb
a
n
ce
• Proton coupling can provide
information about number
of protons.
• Often useful to decouple
protons.
• Irradiate sample with broad
spectrum of frequencies.
• Substituents on C can
enhance of reduce signal.
• Protons enhance the 13C
signal.
2,6
TMS
3,5
1
6
5
O
4
C
180
O
H2
C C
2
4
3
O CH2
CH3
1
160
140
120
100
80
60
40
20
0
11-30
, ppm
Spectrometer
• Magnet
Shim and lock
• Sample probe
Coils and receiver
11-31
NMR imaging
• NMR imaging with a trivalent lanthanide tracer has
been applied to the study of transport and sorption
in ion exchange resins
• The tracer, Gd3+, is a highly effective NMR contrast
agent and an excellent chemical analog for trivalent
actinides
Trivalent lanthanide
7 electrons in f orbital
• Results from these studies can be used to improve
modeling and prediction
11-32
NMR Imaging
• Advantages:
2 and 3-D analysis of heterogeneous granular
structure
Inherently non-invasive probe of spatial structure
Near real-time analysis of static and dynamic
processes
Flexibility to adapt experimental methods to various
sample types and configurations
• Limitations:
Paramagnetic and/or ferromagnetic impurities can
create artifacts and image distortions
Low porosity can lead to long experiment times 11-33
(proton NMR)
Ion-Specific Exchange Resins
• Developed to partition similar inorganic species from
waste streams
Cooperation with French partners in CNAM, ENSCP
• Synthetic organic structures with phenolic functional
groups
• Resorcinol formaldehyde (RF) resins were used in these
experiments (11.5 meq/g dry)
• RF resins were crushed, sieved (80-200 ASTN mesh),
washed, and conditioned to Na+ form
11-34
NMR Flow System
showing evacuation, de-aeration, and over- pressurization systems
Nold
De-aerator
stepper motor
w/ hydraulic
piston for
pressure control
vacuum pump
11-35
Oxford 3T NMR Magnet
with 60 G/cm Imaging Gradient Set
11-36
NMR Basics - spin relaxation
• T1 Relaxation: spin-lattice or longitudinal relaxation of the spin system
Inversion Recovery Curve -T1 Determination
DI Water vs. 0.1 mM Gd Solution
T1 for 0.1mM Gd in Sand is 400ms
0.8
0.6
0.4
1.40
1.35
1.30
1.25
1.20
1.15
1.10
1.05
1.00
0.95
0.90
0.85
0.80
0.75
0.70
0.65
0.60
0.55
0.50
0.45
0.40
0.35
0.30
0.25
0.20
0.15
0.10
0
0.05
0.2
0.00
o
Relative Magnetization-M
z(t)
1
-0.2
-0.4
-0.6
T1 for DI Water in Sand is 1730ms
DI Water
-0.8
0.1 mM Gd Solution
-1
Time (sec)
measured using an inversion recovery sequence where,
11-37
Mz(t) = Mo[ 1 - 2 (exp(-t /T1)) ]
T1 Weighting Experiment - Inversion Recovery
5mm tube of H2O surrounded by 0.1mM Gd solution in sand
Water signal
suppressed
Gd Signal Intensity
Weighted
11-38
Gd Sorption with Phenolic Resin and Sand
8mm diameter by 15mm long sample saturated w/ 1.0 mM Gd
sand/resin
interface
11-39
NMR Imaging Studies of 2-D Flow
1.0 mM Gd into homogenized RF resin and sand sample
Flow direction
1.25cm
0.8 cm
Image 1: water saturated sample
Image 2: 55ml of 1.0mM Gd in
Image 3: 80ml of Gd in
Image 4: 110ml of Gd in
Image 5: 160ml of Gd in
Image 6: 200ml of Gd in
Fingering Flow Phenomenon
11-40
Resin Column data
1.0
0.9
[Gd]out / [Gd]in
0.8
End of Gd Flow
0.7
0.6
0.5
0.4
#6
0.3
#3
0.2
#5
0.1
#4
0.0
0
100
200
300
400
500
600
700
800
900
-0.1
Total Volume Into Column (ml)
11-41
1000
Gadolinium Complexation with Phenolic RF Resin
8mm diameter by 15mm long resin sample saturated with 1.0 mM Gd solution
Cool Spots Showing
Voids and Low
Sorption Sites
Hot Spots Showing
Gd Sorption Sites
11-42