Kaptay / Day 3 / 4
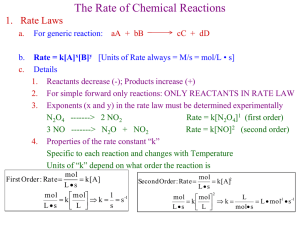
advertisement

See J94
Kaptay / Day 3 / 1
Day 3.
Interfacial energies in high temperature systems
George Kaptay
A 4-day short course
1
Kaptay / Day 3 / 2
Modeling algorithm
Interfacial energies
Interfacial forces
Interfacial phenomena
Complex phenomena
2
Kaptay / Day 3 / 3
Types of phases and interfaces to be modeled
W(s)
gas
NaCl(l)
Al(l)
MgO(s)
Si(s)
AlN(s)
TiC(s)
3
Kaptay / Day 3 / 4
Modeling interfacial energies
A
B
A/ B
G A / B
A/ B
The excess interfacial Gibbs energy:
GA / B G
A
A/ B
G
B
A/ B
G A / B H A / B T S A / B
4
See J48
Kaptay / Day 3 / 5
The excess interfacial enthalpy
for the liquid/gas interface
H lA/ gB
zbA ziA
o
o
U lA A U lA
zbA
For liquid metals (structures surface – see Day 1 / 18):
(11-9)/11 = 0.182
Where the cohesion energy of the liquid metal (classic):
U lAo v H Ao R T
5
See J48
The excess interfacial entropy for the
liquid/gas interface
Kaptay / Day 3 / 6
S sA / g
Vi
R ln
Vb
From the LEED measurements [Somorjai et al]:
S sA / g
u
R ln
u
2
2
b
1/ 2
R ln
b
SlA/ gB S sA / gB 4 1 J / molK
6
See J48
The molar surface area of the liquid/gas interface
Kaptay / Day 3 / 7
4
3 N Av
Vm r
3
fb
N Av
r
fi
2
f Vm N Av
2/3
1/ 3
3 f b
f
4
2/3
1/3
fi
For the {111} plane of the fcc crystal: fi = 0.906.
For fcc crystals fb = 0.740 → f = 1.09.
When an fcc crystal is melted, ΔmV = 1.06, → f = 1.06.
7
See J48
Surface tension of pure liquid metals
Kaptay / Day 3 / 8
lAo / g
0,182 v H Ao (5,5 1) T
1,06 V
o 2/3
A
N 1Av/ 3
Experimental points
Calculated values
8
Kaptay / Day 3 / 9
Surface tension of liquid metals / new age (1)
HlA/ gB A H
H H
o
c,i
o
c,i ,m
H
(11-9)/11 = 0.182
o
c ,i
C (T T )
o
p ,i
o
c,i ,m
H
o
m,i
C (T
o
p,i
o
cr,i
o
c ,i ,Tcr ,i
0
T )
o
m,i
To correlate the calculated values:
H
o
c ,i , m
q1 R T
o
m,i
q R T
2
o 2
m,i 9
Kaptay / Day 3 / 10
From vaporization enthalpy
160000
Rb
Na
K
120000
Cs
Hg
80000
-H
o
c,i,m
J/mol
q1 = 25.4 ± 1.2, q2 = 0.
Li
40000
From critical points
0
0
1000
2000
. o
RT
H
o
c ,i , m
m ,i
q1 R T
o
m,i
3000
4000
J/mol
q R T
2
o 2 10
m,i
Kaptay / Day 3 / 11
Surface tension of liquid metals / new age (2)
3 fb
f
4
2/3
1/ 3
fi
For the {111} plane of the fcc crystal: fi = 0.906.
For fcc crystals fb = 0.740 → f = 1.09.
For melted fcc crystals fb,fcc = 0.74/(1.12) = 0.66 → f =1.01
For melted bcc crystals fb,bcc = 0.68/(1.096) = 0.62 → f=0.97
For melted hcp crystals fb,hcp = 0.74/(1.086) = 0.68 → f=1.03
11
Average for melted fcc, bcc and hcp crystals: f = 1.00 ± 0.02
Kaptay / Day 3 / 12
Surface tension of liquid metals / new age (3)
S
S
o
i/ g
o
vib i / g
S
o
vib i / g
ord S
o
i/g
S
o
i/g
5 1J / molK
S 6 2J / molK
o
m i
S
o
vib i / g
H H
o
c,i
ord S
o
i/g
o
c,i ,m
ord S
o
i/g
1 3
C (T T )
o
p ,i
o
m,i
12
Kaptay / Day 3 / 13
Surface tension of liquid metals / new age (4)
o
i,m
(0.182 0.02) H
H
(1 3) T
o
c,i , m
o 2/3
N
(1.00 0.02) Vi , m
o
c ,i , m
q1 R T
o
m,i
o
m,i
1/ 3
Av
q R T
o 2
m,i
2
q1 = 25.4 ± 1.2, q2 = 0.
o
i,m
(38 10)
o
m,i
T
V N
o 2/3
i,m
1/ 3
Av
13
Kaptay / Day 3 / 14
1
4
Ga
2
0.8
2
i,m, exp, J/m
3.5
i,m, exp, J/m
In Sn
3
0.6
Hg
Ge
0.4
Bi
Sb
0.2
Re
0
2.5
0
0.2
0.4
0.6
0.8
2
i,m, calc, J/m
1
1.2
Ir
Fe
W
Co
2
Ni
Ta
V
1.5
Pt
Al
Zr
1
Tl
0.5
Cu
Ag
K Na
Sr
0
Au
Pb
Hf
Ca
Cs
0
0.5
1
1.5
2
2.5
i,m, calc, J/m2
3
q1 = 26.3 and q2 = -2.62.10-4 molK/J
3.5
4
14
Kaptay / Day 3 / 15
T-coefficient of surface tension / new age (5)
o
i
q1 R T q2 R T
1
o 2/3
i,m
fV
o
i
o ,T
i
C T T S
T T N
o 2
m,i
o
m,i
o
i,m
o
i
o,T
i
(0.182 0.026) C
o
m,i
2/3
o
i/g
1/ 3
Av
(T T )
o
p ,i , m
(1 3)
1/ 3
Av
o
m,i
o
m,i
V N
o 2/3
i,m
o
p,i
2 o o
i i , m
3
15
T
Kaptay / Day 3 / 16
0.25
2
T i,m, exp, mJ/Km
T i,m, exp, mJ/Km
2
0.5
0.4
Hg
0.2
0.15
Sn
In
Ni
Ge
Bi
0.1
0.05
Ga
0.3
Co
Sb
0
0
0.03
T
0.06
0.09
2
,
calc,
mJ/Km
i,m
0.12
Cu
Au
0.2
0.15
Ag
Pb
K
0.1
Na
Al
Cs
Tl
Ca
Sr
0
0
0.1
0.2
0.3
i,m, calc, mJ/Km2
T
0.4
0.5
16
Kaptay / Day 3 / 17
The classic model for surface tension was
improved by changing 2 things at the same time:
i. the cohesion energy is made T-dependent through heat
capacity (known for 130 years)
ii. a new, ordering term is taken into account in the excess
surface entropy (known for 30 years)
General lesson: Models exist on almost everything.
Majority of them can be improved. If you change only one
thing in the model, usually you spoil it. You must be brave
enough to change at least two (sometimes three), different
17
things in the model to get it work again – in a better way.
See P75
Surface structure of MX type molten salts
Kaptay / Day 3 / 18
-
+
-
-
+
+
-
+
Vapour
-
+
-
o
VMX
2 (rM rX ) 3 N Av
MX
10 9
0.1
10
+
+
-
Bulk liquid of
MX associates
-
+
o
MX
2 (rM rX ) 2 N Av
f MX 21 / 3 1.26
SlMX / gB SlM / gB 3 2 J / molK
18
Kaptay / Day 3 / 19
Surface tension of MX molten salts
o
MX
/g
o
0,10 v H MX
(3,8 2) T
1,26 V
o
MX
2/3
N 1Av/ 3
Experimental points
Calculated values
19
Kaptay / Day 3 / 20
Surface tension of molten monoxides (MO)
MO
FeO
MgO
CaO
SrO
BaO
o
MX / g
0,10 v H
o
MX
1,26 V
o
MX
vH
T
K
kJ/mol
1.650
2523
1823
1823
1823
413.2
574.9
578.3
477
336.4
(3,8 2) T
2/3
N 1Av/ 3
Vl
MO/g,
cm3/ mJ/m2
mol
model
15.8
532 70
16.5 695 100
21.1
623 90
27.8
425 60
34.1
239 30
MO/g, J/m2
measured
550 50
700 100
650 100
20
250 50
Kaptay / Day 3 / 21
Surface energy of solid metals
o
sA / g ,Tm
1.15
o
lA/ g ,Tm
21
Kaptay / Day 3 / 22
Surface energy of solid metallic mono-carbides
o
sMC / g
M
Ti
Zr
Hf
V
Nb
Ta
W
o
sM / g
VoM
cm3/mol
10,55
14,01
13,41
8,34
10,84
10,87
9,53
V
V
o
M
o
MC
VoMC
cm3/mol
12,15
15,34
15,03
11,49
13,42
13,40
12,42
2/3
s H 717 f H
o
M
2 s H
sHoM
fHoMC
kJ/mol
469,9
608,8
619,2
514,2
725,9
782
849,4
kJ/mol
-184,1
-202,5
-208,4
-101,9
-140,6
-144,0
-40
o
M
sM/g
J/m2
1,88
2,02
2,12
2,15
2,51
2,87
3,11
o
MC
MC/g
J/m2
2,50
2,39
2,45
2,26
2,38
2,45
2,47
22
Kaptay / Day 3 / 23
Surface energy of solid ionic mono-oxides
o
sMO / g
MX
(0,08 0,01) s H
1,26 V
sH,
o
sMO
o
MO
2/3
(3,8 2) T
N
1/ 3
Av
MO/g, J/m2
MO/g, J/m2
T, K
kJ/mol
Vs,
cm3/mol
NaCl
231
27,0
0
0,20 0,02
0,212
MgO
656
11,0
0
1,00 0,12
1,04
CaO
677
16,6
298
0,78 0,10
0,82
SrO
578
21,9
0
0,56 0,07
-
BaO
425
26,7
1373
0,30 0,05
0,29
FeO
523
12,3
0
0,74 0,09
0,732
23
exp
See J67
Excess interfacial enthalpy of sA/lA interface
Kaptay / Day 3 / 24
The Kelvin equation for the critical radius of nucleation:
2 sA / lA m H Ao
rcr
V Ao
The molar volume can be modeled as:
T
1
Tm , A
4
3 N Av
V rA
3
fb
o
A
m H Ao
3.224 rA
sA / lA
1/ 3
o 2/3
fb
rcr
VA
N 1Av/ 3
T
1
T
m
,
A
At T 0 K, the solid nucleus will be stable from an atom, i.e. rcr rA:
H sA / lA
f b1/ 3
m H Ao
3.224
24
Kaptay / Day 3 / 25
Excess interfacial entropy of sA/lA interface
From side of the solid there is not too much change in freedom:
S sAsA/ lB 0
Liquid atoms will loose part of their freedom at the s/l interface.
The entropy of melting:
m S m S vol m S conf m S str
The excess interfacial entropy:
sAo / lA
lB
S sA
/ lB 0,5 ( m S B,vol m S B,conf )
f b1 / 3
T
o
mH A
m S A,vol m S A,conf
3,224
2 1,06
0,986 V
o 2/3
lA
N
1/ 3
Av
25
Kaptay / Day 3 / 26
Interfacial energy sA/lA
exp , mJ/m
2
sAo / lA
f b1 / 3
T
o
mH A
m S A,vol m S A,conf
3,224
2 1,06
0,986 V
o 2/3
lA
N 1Av/ 3
400
300
200
100
0
0
100
200
300
theor, mJ/m
2
400
26
Kaptay / Day 3 / 27
Summary of interfacial energies of
pure metals (example: Fe)
Tm
3
sg
g , J/m
2
2,5
2
lg
1,5
1
sl
0,5
0
0
500
1000 1500
T, K
2000
2500
27
Kaptay / Day 3 / 28
Interfacial energy sA/lB
There is an extra excess enthalpy term, connected with the interaction of
A and B atoms across the interface:
H sA / lB H sAsA/ lB H sAlB / lB (H sAiA / lB H Ab ) (H sAiB / lB H Bb )
( ziA U AA ziAB U AB zbA U AA ) ( ziB U BB ziBA U AB zbB U BB )
1
2 ( zbA ziA ) U AB (U AA U BB )
2
From the theory of regular solutions:
A B
1
zbA U AB (U AA U BB )
2
Finally, the new excess enthalpy term:
H sA / lB 2 A 28AB
Kaptay / Day 3 / 29
f b1, /A3
sA / lB
3,224
Interfacial energy sA/lB
m H 2 lAlB
o
A
T
m S B ,vol m S B ,conf
2 1,06
V V
o 1/ 3
sA
o 1/ 3
lB
2/3
N Av
Zn/Sn (473 K), Ag/Pb (608 K), Cu/Pb (1193 és 1093 K), Fe/Cu (1373
K), Nb/Cu (1773 K), W/Cu (1773 K), Mo/Sn (1873 K), W/Sn (2273
K),
29
Fe/Pb (1373 and 1193 K) and Fe/Ag (1373 K)
Kaptay / Day 3 / 30
Interfacial energy between two immiscible liquids
lA / lB
2 lAlB S lAlB T T
1
lA / g lB / g
Tc
1.26
lA/lB, mJ/m
2
160
120
80
Al-Bi
40
Ga-Pb
0
0
300
600
T
900
1200
1500
Ga-Pb: D.Chatain, L.Martin-Garin, N.Eustathopoulos: J. chim.phys., 79 (1982) 569
30
Al-Bi: I.Kaban, W.Hoyer, M.Merkwitz: Z.Metallkunde, 94 (2003) p.831
Kaptay / Day 3 / 31
Covalent ceramic / liquid metal interface
The London dispersion forces connect the atoms across the interface:
U a a
WsAC / lB
I B Ii
3
A B i
2
I B Ii
A
2r6
3
1
4 sAC lB
B
Cu
Ga
Sn
Pb
Bi
B C
I B IC
B A I B I A
xA
xC
6
6
I
I
I
I
(
r
r
)
(
r
r
)
B
A
B
C
B
A
B
C
WSi/B
model
mJ/m2
22,6
16,4
9,9
5,6
5,2
WN/B
model
mJ/m2
60,5
38,7
20,3
10,0
8,8
WSiN4/B
model
mJ/m2
83,1
55,0
30,2
15,6
14,0
WSiN4/B
exp
mJ/m2
81,4
56,8
36,5
24,8
23,2
31
See J66
Ionic ceramic / liquid metal interface (1)
Kaptay / Day 3 / 32
The ion A induced dipole (in atom B) interaction with each other:
U AB
z A2 e 2 B
4 π ε o (rA rB ) 4
The adhesion energy:
WA / B
z A2 e 2
2 B, g N Av
3 6 B 4 π εo (rA rB ) 4
The corrected adhesion for dipole induced dipole interaction::
W AC / B
z A2 e 2
2 B , g N Av
3 6 B 4 π ε o (rA rB ) 4
4 B , g AC
1
6
6 (rA rB ) 32
Kaptay / Day 3 / 33
Ionic ceramic / liquid metal interface (2)
The corrected adhesion energy for ion the ionic moment of ion C:
W AC / B
2
z
B
,
g
A
1,54 105 k2, AC
B (rA rB ) 4
2 B , g AC
1
6
3 (rA rB )
33
Points from for the liquid Cu / MgO, ZrO2, Al2O3, SiO2 systems
Kaptay / Day 3 / 34
Wettability of solid Fe by molten chlorides
W AC / B
2
zA
B, g
5
2
1,54 10 k , AC
B (rA rB ) 4
2 B , g AC
1
6
3 (rA rB )
WAC/B
mJ/m2
AC/g
B/AC/g
mJ/m2
fok
0,39
66,4
138
121
3,48
0,44
85,0
147
115
0,138
2,62
0,48
101,9
169
114
NaCl/Fe
0,098
1,85
0,52
118,8
114
88
KCl/Fe
0,133
1,36
0,55
133,2
99
70
AC/B
rC
nm
IC/IA
MgCl2/Fe
0,074
4,89
CaCl2/Fe
0,104
BaCl2/Fe
k
Calculated data are confirmed experimentally by Vetiukov et al.
34
Kaptay / Day 3 / 35
Interfacial energy in liquid metal / molten salt systems
W AC / B
2
zA
B, g
5
2
1,54 10 k , AC
B (rA rB ) 4
2 B , g AC
1
6
3 (rA rB )
T.Utigard, J.M.Toguri, T.Nakamura, Metall.Trans. B., 17B (1986) 339
NaF/Bi (1373 K), NaCl/Bi (1373 K), NaF/Pb (1273 K), NaCl/Pb (117335K),
NaF/Ag (1273 K), NaCl/Ag (1273 K), NaF/Cu (1373 K) and NaCl/Cu (1373 K)
Kaptay / Day 3 / 36
Concentration dependence of (Gibbs, 1878)
The surface excess (by definition):
o
B
B xB * xB
o
B
1
B
n
The Gibbs equation:
d i d i
i 1
di R T d ln ai
i R T ln ai
o
i
+Gibbs-Duhem
(for binaries):
aB
R T B d ln a B
o
A
0
36
Kaptay / Day 3 / 37
Langmuir (1918)
The equilibrium between bulk and surface phases:
A* + B = A + B*
ads G o
K exp
RT
x B * a A
K
(1 x B *) a B
For the infinitely diluted solution of B in A: aA = 1, aB= gB xB
b xB
xB *
1 b xB
b K g
B
37
Kaptay / Day 3 / 38
Belton (1976)
b xB
xB *
1 b xB
B xB * xB
o
B
o
B
1
B
xB
b
B
1
B 1 b xB
aB
R T B d ln a B
o
A
0
o
A
R T
B
Integration at infinitely
diluted solution of B:
dlnaB = dxB/xB.
ln(1 b x B ) x B
38
Kaptay / Day 3 / 39
Gibbs – Langmuir – Belton (1878 – 1976)
At infinitely diluted solution of B: xB << 1 (and if b >>1)
o
A
R T
ads G
Ka exp
RT
o
o
B
ln(1 K a aB )
A* + B = A + B*
Ao Ao Bo Bo
K a exp
R T
[??]
39
Kaptay / Day 3 / 40
Theoretical Concentration dependence of Fe-O/g
(1823K ) 1960 291 ln(1 284 CO )
40
Kaptay / Day 3 / 41
Theoretical concentration dependence of Fe-S/g
(1823K ) 1960191 ln(1 523 CS )
41
Kaptay / Day 3 / 42
Additive extension to ternary Fe-O-S system
FeOS / g 1960 291 ln(1 284 aO ) 191 ln(1 523 aS )
Experimental points
Calculated values
42
Kaptay / Day 3 / 43
Taking into account the competition
between O and S atoms for surface sites
A B C
R T
K a , BaB K a ,C aC
o
A
K a , BaB K a ,C aC
ln1 K a , BaB K a ,C aC
o
o
C
B
Experimental points
Calculated values
43
Kaptay / Day 3 / 44
On the Butler equation (1932)
A B / g A / g B / g
A B / g
o
A/ g
R T
o
A
aA *
aB *
R T
o
ln
B / g o ln
aB
aA
B
Compared to the Gibbs equation:
i. it is easier to teach and to apply, but still
ii. at equal assumptions it provides the same results.
The surface activity coefficient as function of surface
44
composition is to be modelled
Kaptay / Day 3 / 45
Modeling surface excess Gibbs energy
R T ln a A * R T ln x A * G
Ei
A
The model is based on the ratio of broken bonds ():
G AEi (1 ) G AE
For liquid metals: Hoar, Melford, 1957: 0.330.50
Monma, Suto, 1961: = 0.16 -0.20,
Speiser, Poirier, Yeum, 1987 – 1989: = 0.25,
Tanaka, Iida, Hara, Hack, 1994 – 2000: = 0.17,
For molten salts: Tanaka et al.: = 0.06, later -0.1 (!!??)
45
Kaptay / Day 3 / 46
The Butler equation, applied to associated liquids
AaBb
o
a A A b B B f H AaBb
a A b B
Al-Ni
Experimental points: V.N.Eremenko, V.I.Nizhenko, N.I.Levi,
46
B.B.Bogatirenko: Ukr. Him. Zh., 1962, vol.28, No.4, pp.500-505
See J99
Surface phase separation in monotectic alloys (a)
A/ g B / g
Kaptay / Day 3 / 47
A = 1 J/m2, B = 0.5 J/m2, VA = 1 10-5 m3/mol, VB = 2 10-5
m3/mol, = 20 kJ/mol. Then: Tc = 1202.79 K.
At T = 875.86 K, bulk separation at xB = 0.1 and xB = 0.9
1
Fig.1.a: XB = 0.00068
A
B
A, B, J/m
2
0,99
0,98
0,97
solution
0,96
0
0,2
0,4
xB*
0,6
0,8
1
Partial surface tensions as function of surface content
47
See J99
Surface phase separation in monotectic alloys (b)
Kaptay / Day 3 / 48
1
Fig.1.b: XB = 0.00072
0,99
A, B, J/m
2
A
B
0,98
0,97
solution
0,96
0
0,2
0,4
xB*
0,6
0,8
1
48
Partial surface tensions as function of surface content
See J99
Surface phase separation in monotectic alloys (c)
Kaptay / Day 3 / 49
1
Fig.1.c: XB = 0.000755
A, B, J/m
2
0,99
A
B
0,98
0,97
2 solutions
0,96
0
0,2
0,4
xB*
0,6
0,8
1
49
Partial surface tensions as function of surface content
See J99
Surface phase separation in monotectic alloys (d)
Kaptay / Day 3 / 50
1
Fig.1.d: XB = 0.00080
A, B, J/m
2
0,99
solution
A
0,98
0,97
B
0,96
0
0,2
0,4
xB*
0,6
0,8
1
50
Partial surface tensions as function of surface content
See J99
Surface phase separation in monotectic alloys (e)
Kaptay / Day 3 / 51
1
Fig.1.e: XB = 0.00085
A, B, J/m
2
0,99
A
0,98
solution
0,97
B
0,96
0
0,2
0,4
xB*
0,6
0,8
1
51
Partial surface tensions as function of surface content
See J99
Surface phase separation in monotectic alloys (f)
Kaptay / Day 3 / 52
0
logxB*
-1
-2
-3
-4
-6
-5
-4
-3
-2
-1
logxB
Surface composition as function of bulk composition 52
0
See J99
Surface phase separation in monotectic alloys (g)
Kaptay / Day 3 / 53
1.2
, J/m
2
1
0.8
0.6
0.4
0.2
0
-6
-5
-4
-3
-2
-1
0
logxB
Surface tension as function of bulk composition
53
See J99
Surface phase separation in monotectic alloys (h)
Kaptay / Day 3 / 54
1400
1200
1 bulk liquid
*
cr
T
T, K
1000
800
SPT line
Tcr
1 bulk liquid
+ nanolayer
600
2 bulk liquids
400
200
0
-6
Fig.2.c
-5
-4
-3
-2
-1
logxB
A phase diagram with a surface phase separation line54
0
See J99
Surface phase separation in monotectic alloys (i)
Kaptay / Day 3 / 55
T=800 K
d /dT 104, J/m2K
8
6
T=986 K
4
2
T=1200 K
0
-2
-4
-3.5
-3
-2.5
-2
-1.5
-1
logxB
55
T-coefficient of surface tension as function of bulk composition
Kaptay / Day 3 / 56
Two shapes of welding pools
1400
1200
1 bulk liquid
T
T, K
1000
800
SPT line
Tcr
*
cr
1 bulk liquid
+ nanolayer
600
2 bulk liquids
400
200
0
-6
-5
-4
-3
-2
-1
0
logxB
d
0
dT
d
0
dT
56
Thank you for your attention
57