View Poster - Idaho EPSCoR
advertisement

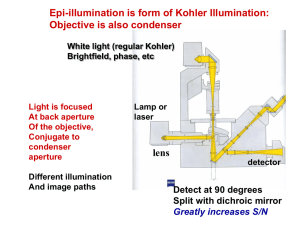
Energy Transfer of Fluorescent CdSe/ZnS Quantum Dots and Gold Nanoparticles and Its Applications for Mercuric (II) Ion Detection By Ming Li and Nianqiang Wu WVNano Initiative, Department of Mechanical and Aerospace Engineering at West Virginia University Outline Background and Objectives Sensor Design Size Effect on Energy Transfer Mercuric (II) Detection Conclusions Background Förster resonance energy transfer (FRET) is a process that occurs between an excited molecular donor and a molecular acceptor; Bio/environmental monitoring Therapeutics/diagno stics Energy Transfer Photovoltaic devices Light emitters The rate of FRET energy transfer strongly depends on the spectral overlap of the emission spectrum of the donor with the absorption spectrum of the acceptor . Acceptors include conventional organic dyes such as , Fluorescein-5-isothiocyanate (FITC), , Cy3 , etc. Cy5 Ultrafine gold nanoparticles quench the fluorescence emission much efficiently, following nanometal surface energy transfer (NSET). Large sized gold nanoparticles exhibit size-tunable localized surface plasmon (LSPR), and the additional electromagnetic field should affect energy transfer in a different manner. Objectives The overall objective is to develop an ultrasensitive strategy for trace mercuric (II) detection based on the energy transfer between CdSe/ZnS quantum dots and gold nanoparticles. Specific aims are as follows: (1) Investigating size effect of gold nanoparticles on fluorescence quenching of CdSe/ZnS quantum dots; (2) Gaining mechanistic insights into the energy transfer mechanism; (3) Developing the sensor for trace mercuric (II) ion detection. Sensor Design Energy transfer QD gold spacer Used DNA sequence: 1): 5’-HS-CAGTTTGGAC-3’ 3’-GTCTTTCCTG-SH-5’ 2): 5’-HS-CAGCAGGACTTTGGACCAAC-3' 3'-GTCGTCCTGTTTCCTGGTTG-SH-5’ 3): 5’-HS-CAGCTGGACCGTAGTTTGGACCTACGTACG-3' 3'-GTCGACCTGGCATCTTTCCTGGATGCATGC-SH-5’ CdSe/ZnS quantum dots as the energy donor and gold nanoaprticles as the energy acceptor; Change the size of gold nanoparticles and the space between quantum dots and gold nanoparticles by using different DNA lengths. Size Effect on Energy Transfer (a) (b) 20 nm (a) Absorption and fluorescence emission spectra of the used quantum dots (b) TEM image of the used quantum dots The quantum dots exhibit a fluorescence emission band at 570 nm and a particle size of 3.4 nm in diameter. (a) (b) 3 nm gold nanoparticles show no observable LSPR absorption; 10 nm (d) (c) 15 nm and 80 nm gold nanoparticles have LSPR peaks at 520 nm and 550 nm, respectively; The spectra overlap increases as the increasing particle size. 50 nm 50 nm (a) Extinction spectra of 3, 15 and 80 nm gold nanoparticles and fluorescence emission spectra of DNA-functionalized quantum dots. (b) TEM images of 3, 15 and 80 nm sized gold nanoparticles, respectively. Fluorescence quenching by different sized gold nanoparticles (a) (b) 3 nm 15 nm 80 nm (c) Quenching of fluorescence emission of the quantum dots by the gold nanoparticles with different sizes. (a) fluorescence emission spectra, (b) emission intensity at 570 nm and (c) Stern-Volmer plots as a function of mercuric (II) ion concentration. (a) (b) (c) Experimental data points and theoretical curves of the quenching efficiency versus the separation distance. • The quenching efficiency increases as the increasing particles size; • 3 nm gold nanoparticles quench fluorescence emission following the NSET mechanism with a 1/d4 distance dependence; • 15 nm and 80 nm gold nanoparticles quench fluorescence emission following the FRET mechanism with a 1/d6 distance dependence. Mercuric (II) Detection Excitation Au + QD Au emission Hg2+ ( ) • Increasing the DNA loading on quantum dots; • A quantum dot coupled to several gold. Energy transfer Excitation GTCTTTCCTG-S QD S-CAGTTTGGAC no emission Au (a) (b) (c) • Fluorescence emission intensity at 570 nm decreases with the increasing mercuric (II) concentration; • The detection limit is down to 0.2 nM. (a) Fluorescence emission spectra and (b) quenching efficiency of quantum dots as a function of mercuric (II) concentration; (c) is the linear region of (b) at low concentration. Fluorescence quenching efficiency in the presence of various metal ions. The concentration of each metal ion is 100 nM (96 nM QDs, 104 nM Au NPs and 0.1 mM ethylenediamine in 0.3 M PBS). Conclusions • The quenching efficiency of fluorescence emission of quantum dots increases as the increasing particle size; • The 3 nm gold nanoparticles quench fluorescence emission following the NSET mechanism with a 1/d4 distance-dependence; • The 15 nm and 80 nm gold nanoparticles quench fluorescence emission following the FRET mechanism with a 1/d6 distancedependence; • The increasing quenching efficiency with the increasing gold particle size is attributed to the enhanced LSPR and the increasing spectral overlap; • The quantum dot/DNA/gold sensor exhibits an ultrasensitive detection toward mercuric (II) and high selectivity over other environmental ions. Acknowledgments This work was supported by an NSF grant (CBET-0754405). The resource and facilities used were partially supported by an NSF RII grant (EPS 1003907) and a Research Challenge Grant from the State of West Virginia (EPS08-01), the West Virginia University Research Corporation and the West Virginia EPSCoR Office.