Lecture 7
advertisement

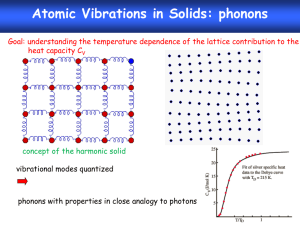
Thermal Behavior – II Free Energy & Phase Diagrams [partly based on Chapter 7, Sholl & Steckel] • Free energy changes – – – • • excluding vibrational entropy of solids including just the harmonic vibrational part including the quasi-harmonic/anharmonic contribution Case studies Also see: The Free Energy • • • • • • • • At non-zero temperatures, we need to consider the free energy Gibbs free energy: G = E – TS + PV Helmholtz free energy: F = E – TS E is the ground state (zero temperature) energy which can be computed using DFT The entropy: S = Selec + Slattice (+ Sconfig) Selec is the electronic contribution to entropy; easy to calculate but is generally small (depends on the situation and our goal) Slattice is very difficult to compute as it involves treatment of phonons (more later) Sconfig is the configurational entropy which can be used when appropriate Oxidation • When is a metal unstable to oxidation? • In other words, under what conditions is the following reaction favorable: M + (x/2)O2 MOx • … when DG is negative DG = GMOx – [GM + (x/2)GO2] DG = EMOx – EM – (x/2)GO2 – T(SMOx – SM) + P(VMOx – VM) • If P = 0 and SMOx = SM DG = EMOx – EM – (x/2)GO2 DG = EMOx – EM – x mO = [EMOx– (x/2)mO2] – EM DFT energies O chemical potential (depends on T & P) • Note that the TS and PV terms are retained for oxygen, and hence the T & P dependence of DG is preserved Stability of Oxides DG = [EMOx– (x/2)mO2] – EM • [EMOx– (x/2)mO2] and EM may be plotted versus mO2 for various choices of x (i.e., various types of oxides) M3O2 not stable M2O stable above this chemical potential (But what is this?) The O Chemical Potential [See Hill, Statistical Thermodynamics, Chapter 8 for details] Zero K DFT energy for isolated O2 molecule Chemical potential at temperature T and some reference pressure p0 (this includes translational, rotational, vibrational and electronic contributions, and can be computed using statistical mechanics without any fitting to experimental data) • Thus the (T, P) combination that corresponds to the critical O chemical potential of the previous slide may be determined • This will provide us with a phase diagram for the equilibrium between the metal and its oxide Phase (Ellingham) Diagram • Qualitatively correct, but large quantitative errors to be expected • Sources of errors – DFT approximations – Neglect of the vibrational entropy of the solids involved Surface Phase Diagrams • Ag is a good catalyst for many reactions involving O • It was later realized that it was a thin layer of surface oxide that was catalytically active, rather than pure Ag itself • DFT work shows that under the catalytically active conditions a surface oxide is thermodynamically favored The Free Energy Revisited • • • • For the solid, G = E – T(Selec + Slattice) + Ezero-pt + PV E is the energy we have been talking about so far. This is the total (internal) energy of the electron-ion system The electronic entropy Selec(T,V) can also be easily calculated Well known statistical mechanics formulae available for Sel(T,V), which is written in terms of the electronic “density of states” hcpbcc transition The Free Energy: Lattice part • • • • G = E – T(Selec + Slattice) + Ezero-pt + PV Slattice is the entropy due to lattice vibrations Before we get to lattice vibrations, let us consider “normal modes” A simple illustration … going back to simple harmonic oscillators • • Just one mode or frequency of vibration Two modes or frequencies of vibration • • • r(w) is called the vibrational density of states What is a “mode”? If the system is perturbed so that one of the modes is excited, then the system will display periodic vibration at the frequency corresponding to that mode If the system is arbitrarily perturbed, the vibration that results will not be periodic with any particular frequency, but will be a linear combination of the modes For N masses, we will have N modes or frequencies In general, as N is large (like in a solid), we will have a continuum of modes/frequencies, resulting in the phonon band structure or spectrum (very similar to electronic band structure) Phonons • Just like electronic states in a periodic lattice, the lattice vibrational modes may be classified using a k-point within the 1st BZ • Thus, if there are N atoms within a periodically repeating unit cell (in 3-d), there will be a total of 3N vibrational modes per k-point, each with a distinct frequency wik (i = 1-3N, k = a point in the 1st BZ) • Out of the 3N modes, 3 will be acoustic and the others will be optical “branches” • The low frequency (or long wavelength) portion of the acoustic branches are essentially elastic acoustic waves which travel at the velocity of sound in that material • The modes of the optical branch may result in time-varying dipoles, and hence can be excited by electromagnetic (or optical) waves • Phonons: Quantized lattice waves • For an interesting applet, see: http://dept.kent.edu/projects/ksuviz/leeviz/phonon/phonon.html Phonons in Bulk Silicon The Free Energy: Lattice part (contd.) E zero pt TS lattice harmonic 0 1 r (w ) w kT ln (1 e 2 w / kT d w Vibrational density of states • • • • • G = E – T(Selec + Slattice) + Ezero-pt + PV Computation of the vibrational contribution to the free energy boils down to the determination of the phonon band structure of the system, which is extremely computationally demanding Note: For a single harmonic oscillator, the density of states is just the Dirac delta function The above picture is still not complete, as it includes only the harmonic contribution to vibrations The anharmonic contribution is necessary to implicitly include thermal expansion, which will alter the vibrational contribution for increasing temperatures The Quasi-harmonic Approximation • Within the harmonic approximation – G(T) = E – T[Selec(T) + Slattice(T)] + Ezero-pt – That is, there is no volume dependence! – Phonon frequencies are determined at the equilibrium volume • In the quasi-harmonic approximation, the phonon problem is solved at several choices of the unit cell volume, and the following free energy is evaluated – – – – G(V,T) = E(V) – T[Selec(V,T) + Slattice(V,T)] + Ezero-pt(V) + PV Note the explicit dependence of G on V For each T, the V corresponding to the minimum G is determined This will give the T dependence of V, and hence the thermal expansion The Quasi-Harmonic Approximation A Schematic • For each choice of unit cell volume, compute G as a function of T • Then, at each T, the V corresponding to the minimum G is determined Separate phonon calculation for each column V1 Minimization of G for each choice of T T1 V2 … VN G(V1,T1) T2 TN G(VN,TN) Once the correct volume for a given T is known, the phonon results for that volume may be used to determine the free energy for that phase, and can be compared to the free energy of other competing phases to determine the phase diagram Phase transformations involving solids Experiments Boron Nitride Kern et al, PRB 59, 8551 (1999) Tin Pavone et al, PRB 57, 10421 (1998) The Einstein crystal • • • • • • • To understand the main aspects/reasons of solid-solid transformations such as fccbcc, we can make some simplifying assumptions We assume that the entire fcc lattice has only one characteristic phonon frequency (or mode) Lets assume that the bcc lattice too has just one phonon frequency, but smaller than that of the fcc lattice Lets further assume that the energy Efcc < Ebcc Then: Gfcc = Efcc + 0.5ħwfcc + kTln[1-exp(-ħwfcc/kT)] At low T, fcc will be more stable, however, at high T, as wbcc is smaller than wfcc, the phonons in bcc are easier to excite, resulting in a larger free energy drop in bcc for entropic reasons DFT expt. DFT expt. Phase transformations involving melting Phase diagram of elemental Mg Experimental results Experimental result • • Note: easy to consider high pressure using computational methods; sometimes computation may be the only option Calculation of alloy phase diagrams is considerably more complex: composition variable(s) need to be included (makes calculation laborious) & configurational entropy needs to be added (easy) Extreme pressures • Extreme geophysical pressures may be difficult to create in the lab, but can be simulated easily Extreme pressures – contd. Liquid Solid Extreme pressures – contd. Thermal expansion • Can a material contract when heated? Term Paper Guidelines • • • • • Presentations on December 9 (Friday) Choose topic preferably close to your research DFT has to be a necessary major component of term paper Actual computations are optional Without actually doing any elaborate calculations, come up with a “case” or a “proposal” for doing calculations that could go well with your own primary research topic, which ideally may be pursued after this course – – – – • • Identify a specific problem, and explain why this is important Do a thorough literature search to find out what is already known (experimentally & computationally) – in other words, educate me and the class! Explain the specific DFT strategies undertaken in the past, and the results and insights that have already emerged (this should be the main component of the term paper) Identify a couple of open issues that may be worth pursuing in the future Finalize topic by November 16 Suggested journals for literature search: Phys. Rev. B, Phys. Rev. Lett., Appl. Phys. Lett., J. Appl. Phys., J. Phys. Chem., Nano Letters, etc., within the last 10 years. Term Paper Topic Suggestions • • • • • • • • • • • • • Modeling of amorphous materials (metals, insulators, polymers) Point defects in semiconductors and insulators Metal-oxide interfaces (electronic properties, phase equilibria, adhesion, etc.) Materials for phase change memory applications Materials for hydrogen storage Ferroelectricity/multiferroicity in thin films, multi-layers, etc. Catalytic chemical reactions Materials under extreme conditions (pressure, temperature, radiation, etc.) Magnetism in bulk and nanostructures Modeling of crystal growth (metals, insulators, semiconductors) Methods for computing the dielectric constant of materials Methods for handling the “band gap” and “defect state” problems within traditional DFT Methods for computing energy offsets (e.g., work function, electron affinity, ionization potential, Schottky barrier heights, etc.) Note: The above suggestions are quite broad. You need to choose a specific subtopic within these