NJU09053
advertisement
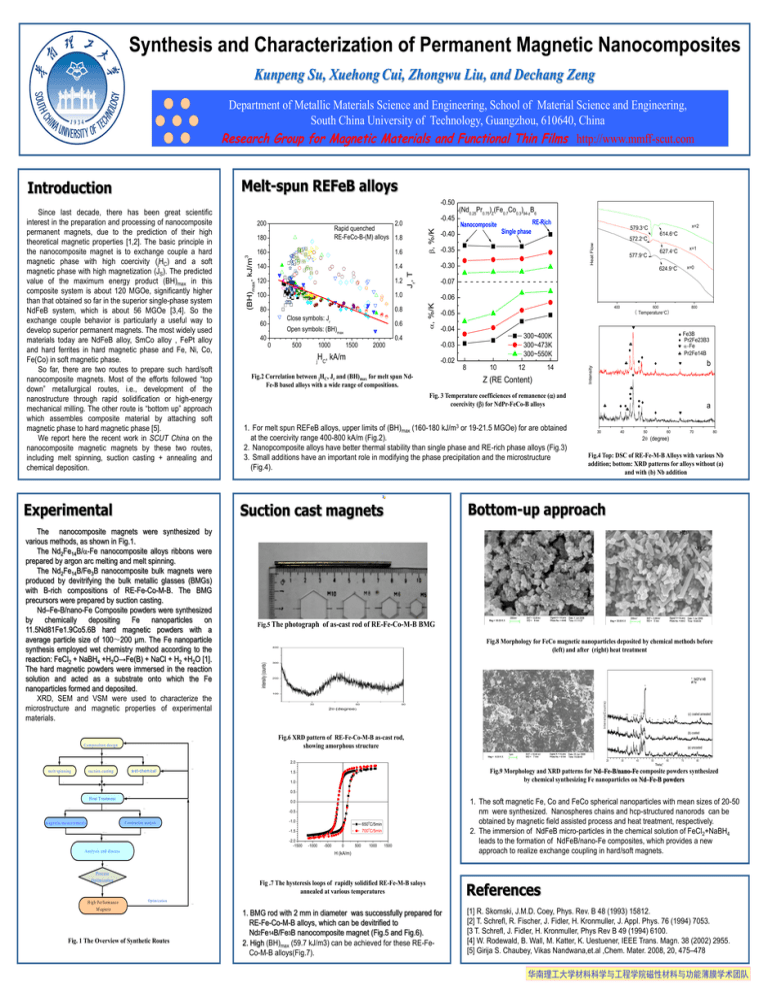
Synthesis and Characterization of Permanent Magnetic Nanocomposites Kunpeng Su, Xuehong Cui, Zhongwu Liu, and Dechang Zeng Department of Metallic Materials Science and Engineering, School of Material Science and Engineering, South China University of Technology, Guangzhou, 610640, China Research Group for Magnetic Materials and Functional Thin Films http://www.mmff-scut.com Melt-spun REFeB alloys Introduction -0.50 Experimental 1.6 140 1.4 120 1.2 100 1.0 80 0.8 Close symbols: Jr 60 40 P -27 C om position design P -1 0 500 m agnetic m easurem ents w et-chem ical P-22 P-16 577.9C 624.9C x=0 -0.06 400 -0.04 300~400K 300~473K 300~550K -0.02 8 Fig.2 Correlation between jHC, Jr and (BH)max for melt spun NdFe-B based alloys with a wide range of compositions. 800 ( TemperatureC) -0.03 2000 600 -0.05 10 12 14 Z (RE Content) 1. For melt spun REFeB alloys, upper limits of (BH)max (160-180 kJ/m3 or 19-21.5 MGOe) for are obtained at the coercivity range 400-800 kA/m (Fig.2). 2. Nanopcomposite alloys have better thermal stability than single phase and RE-rich phase alloys (Fig.3) 3. Small additions have an important role in modifying the phase precipitation and the microstructure (Fig.4). Fe3B Pr2Fe23B3 Fe Pr2Fe14B b Fig. 3 Temperature coefficiences of remanence () and coercivity () for NdPr-FeCo-B alloys 30 40 50 a 60 70 80 2 (degree) Fig.4 Top: DSC of RE-Fe-M-B Alloys with various Nb addition; bottom: XRD patterns for alloys without (a) and with (b) Nb addition Bottom-up approach ←HCP ↖FCC Fig.5 The photograph of as-cast rod of RE-Fe-Co-M-B BMG Fig.8 Morphology for FeCo magnetic nanoparticles deposited by chemical methods before (left) and after (right) heat treatment intensity (counts) 400 300 200 100 30 60 90 2(degree) Fig.6 XRD pattern of RE-Fe-Co-M-B as-cast rod, showing amorphous structure Fig.9 Morphology and XRD patterns for Nd–Fe-B/nano-Fe composite powders synthesized by chemical synthesizing Fe nanoparticles on Nd–Fe-B powders P -20 1.5 1.0 P-9 0.5 J (T) P -23 P -14 0.0 -0.5 -1.0 C onstruction analysis P-15 x=1 627.4C -0.07 H , kA/m j C P -7 P -13 572.2C 2.0 P -7 P-12 1500 614.6C P-5 suction casting H eat T reatm ent 1000 x=2 579.3C Single phase -0.35 0.4 P -1 P-8 -0.40 RE-Rich -0.30 0.6 Open symbols: (BH)max Nanocomposite Intensity (BH)max, kJ/m 160 Jr, T 180 , %/K Rapid quenched RE-FeCo-B-(M) alloys 1.8 (Nd0.25Pr0.75)Z(Fe0.7Co0.3)94-zB6 Heat Flow 2.0 Suction cast magnets The nanocomposite magnets were synthesized by various methods, as shown in Fig.1. The Nd2Fe14B/-Fe nanocomposite alloys ribbons were prepared by argon arc melting and melt spinning. The Nd2Fe14B/Fe3B nanocomposite bulk magnets were produced by devitrifying the bulk metallic glasses (BMGs) with B-rich compositions of RE-Fe-Co-M-B. The BMG precursors were prepared by suction casting. Nd–Fe-B/nano-Fe Composite powders were synthesized by chemically depositing Fe nanoparticles on 11.5Nd81Fe1.9Co5.6B hard magnetic powders with a average particle size of 100~200 µm. The Fe nanoparticle synthesis employed wet chemistry method according to the reaction: FeCl2 + NaBH4 +H2O→Fe(B) + NaCl + H2 +H2O [1]. The hard magnetic powders were immersed in the reaction solution and acted as a substrate onto which the Fe nanoparticles formed and deposited. XRD, SEM and VSM were used to characterize the microstructure and magnetic properties of experimental materials. m elt spinning -0.45 , %/K 200 3 Since last decade, there has been great scientific interest in the preparation and processing of nanocomposite permanent magnets, due to the prediction of their high theoretical magnetic properties [1,2]. The basic principle in the nanocomposite magnet is to exchange couple a hard magnetic phase with high coercivity (jHC) and a soft magnetic phase with high magnetization (JS). The predicted value of the maximum energy product (BH)max in this composite system is about 120 MGOe, significantly higher than that obtained so far in the superior single-phase system NdFeB system, which is about 56 MGOe [3,4]. So the exchange couple behavior is particularly a useful way to develop superior permanent magnets. The most widely used materials today are NdFeB alloy, SmCo alloy , FePt alloy and hard ferrites in hard magnetic phase and Fe, Ni, Co, Fe(Co) in soft magnetic phase. So far, there are two routes to prepare such hard/soft nanocomposite magnets. Most of the efforts followed “top down” metallurgical routes, i.e., development of the nanostructure through rapid solidification or high-energy mechanical milling. The other route is “bottom up” approach which assembles composite material by attaching soft magnetic phase to hard magnetic phase [5]. We report here the recent work in SCUT China on the nanocomposite magnetic magnets by these two routes, including melt spinning, suction casting + annealing and chemical deposition. o 650 C/5min o 700 C/5min -1.5 P-16 -2.0 -1500 P-15 A nalysis and discuss -1000 -500 0 500 1000 1500 H (kA/m) 1. The soft magnetic Fe, Co and FeCo spherical nanoparticles with mean sizes of 20-50 nm were synthesized. Nanospheres chains and hcp-structured nanorods can be obtained by magnetic field assisted process and heat treatment, respectively. 2. The immersion of NdFeB micro-particles in the chemical solution of FeCl2+NaBH4 leads to the formation of NdFeB/nano-Fe composites, which provides a new approach to realize exchange coupling in hard/soft magnets. P -18 Process O ptim ization Fig .7 The hysteresis loops of rapidly solidified RE-Fe-M-B saloys annealed at various temperatures P -19 H igh Perform ance M agnets References O ptim ization Fig. 1 The Overview of Synthetic Routes P -20 1. BMG rod with 2 mm in diameter was successfully prepared for RE-Fe-Co-M-B alloys, which can be devitrified to Nd2Fe14B/Fe3B nanocomposite magnet (Fig.5 and Fig.6). 2. High (BH)max (59.7 kJ/m3) can be achieved for these RE-FeCo-M-B alloys(Fig.7). [1] R. Skomski, J.M.D. Coey, Phys. Rev. B 48 (1993) 15812. [2] T. Schrefl, R. Fischer, J. Fidler, H. Kronmuller, J. Appl. Phys. 76 (1994) 7053. [3 T. Schrefl, J. Fidler, H. Kronmuller, Phys Rev B 49 (1994) 6100. [4] W. Rodewald, B. Wall, M. Katter, K. Uestuener, IEEE Trans. Magn. 38 (2002) 2955. [5] Girija S. Chaubey, Vikas Nandwana,et.al ,Chem. Mater. 2008, 20, 475–478 华南理工大学材料科学与工程学院磁性材料与功能薄膜学术团队










