powerpoint - School of Chemical Sciences
advertisement

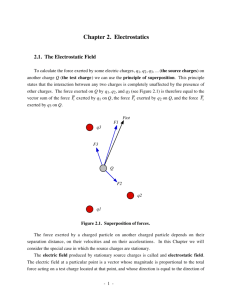
Lecture 23
Born-Oppenheimer approximation
(c) So Hirata, Department of Chemistry, University of Illinois at Urbana-Champaign. This material has
been developed and made available online by work supported jointly by University of Illinois, the
National Science Foundation under Grant CHE-1118616 (CAREER), and the Camille & Henry Dreyfus
Foundation, Inc. through the Camille Dreyfus Teacher-Scholar program. Any opinions, findings, and
conclusions or recommendations expressed in this material are those of the author(s) and do not
necessarily reflect the views of the sponsoring agencies.
The Born-Oppenheimer
approximation
For polyatomic molecules, we use
approximate separation of variables
between the nuclear and electronic variables.
We first solve the electronic Schrödinger
equation, in which nuclei are held fixed.
Next, we solve the nuclear Schrödinger
equation.
The BO approximation introduces important
chemical concepts such as potential energy
surfaces, equilibrium geometries, binding
energies, etc.
The molecular Hamiltonian
The molecular Hamiltonian is …
n
N
2
2
N n
Z
Z
e
Z
e
Hˆ = -å
Ñ2e - å
Ñ 2N + å
+ å I J - åå I
i
I
i=1 2me
I =1 2mN
i< j 4pe 0 rij
I <J 4pe 0 rIJ
I
i 4pe 0 rIi
2
i
Kinetic
(electron)
n
2
e2
N
I
Kinetic
(nuclei)
Coulomb
(e – e)
Coulomb
(n – n)
Coulomb
(e – n)
There is no hope that we can solve the
Schrödinger equation with this Hamiltonian
exactly. Can we separate variables
approximately?
Separation of the Hamiltonian
He(r)
n
N
2
2
N n
Z
Z
e
Z
e
Hˆ = -å
Ñ2e - å
Ñ 2N + å
+ å I J - åå I
i
I
i=1 2me
I =1 2mN
i< j 4pe 0 rij
I <J 4pe 0 rIJ
I
i 4pe 0 rIi
2
i
Kinetic
(electron)
n
2
e2
N
I
Kinetic
(nuclei)
Coulomb
(e – e)
Hn(R)
Coulomb
(n – n)
Coulomb
(e – n)
Separation of the wave function
The first step of (approximate) separation of
variables: assumption of product form of
wave function
Y(r,R) = Y e (r)Y n (R)
Electronic
coordinates
Electronic
wave function
Nuclear
coordinates
Nuclear wave
function
Separation of the wave function
The second step: substitution into the
Schrödinger equation.
resists separation
éë H e (r) + H n (R) ùû Y e (r)Y n (R) = H e ( Y eY n ) + Y e ( H nY n )
= EY e (r)Y n (R)
n
n
N
n
2
Z
e
Hˆ e = - å
Ñ 2e + å
- åå I
i
i=1 2me
i< j 4pe 0 rij
I
i 4pe 0 rIi
2
e2
i
N
Hˆ n = - å
2
I =1 2mN
Ñ 2N
I
N
I
Z I Z J e2
+å
I <J 4pe 0 rIJ
involves also
nuclear coordinates
Separation of the wave function
… we must demote some variables to
parameters.
parameters R are held
fixed
éë H e (r;R) + H n (R) ùû Y e (r;R)Y n (R) = Y n ( H eY e ) + Y e ( H nY n )
= EY e (r;R)Y n (R)
n
n
N
n
2
Z
e
Hˆ e = - å
Ñ 2e + å
- åå I
i
i=1 2me
i< j 4pe 0 rij
I
i 4pe 0 rIi
2
e2
i
N
Hˆ n = - å
2
I =1 2mN
Ñ 2N
I
N
I
Z I Z J e2
+å
I <J 4pe 0 rIJ
nuclear coords. are
considered parameters
Parameters versus variables
Parameters are arguments of a function
with which no differentiation or integration
is performed. For example, the electron
mass is a parameter. Parameters are
held fixed.
Variables are arguments of a function with
which differentiation or integration is
performed. For example, electron
coordinates in the hydrogenic Schrödinger
equation are variables. Variables do vary.
Separation of the wave function
The third step: divide the whole equation by
wave function.
1
1
H eY e ) +
H nY n ) = E
(
(
Ye
Yn
( )
Ee R
Electronic
structure
Nuclear
dynamics
Separation
achieved
En
Hˆ e ( r;R ) Y e ( r;R ) = Ee ( R ) Y e ( r;R )
{
}
Hˆ n ( R ) Y n ( R ) = E - Ee ( R ) Y n ( R )
The Born-Oppenheimer
approximation
The BO approximation breaks the original
problem into two smaller problems that must
be solved in sequence:
Electronic structure (nuclei held fixed)
2 ü
2
n
N n
ìï n 2
Z
e
e
ï
2
I
Ñe + å
- åå
í- å
ý Y e ( r;R ) = Ee ( R ) Y e ( r;R )
i
i< j 4pe 0 rij
I
i 4pe 0 rIi ï
ïî i=1 2mei
þ
Nuclear dynamics
2
2
N
ìï N
üï
ZI ZJ e
2
ÑN + å
+ Ee ( R ) ý Y n ( R ) = EY n ( R )
í- å
I
2m
I
=1
I <J 4pe 0 rIJ
NI
ïî
ïþ
Potential energy surface (PES)
The Born-Oppenheimer
approximation
In the electronic Schrödinger equation,
nuclear coordinates are parameters and
their kinetic energy operator does not act on
them; the nuclei are held fixed. This is
justified by that a nucleus is 1800+ times
heavier than an electron and sluggish.
The electronic Schrödinger equation must be
solved for various nuclear positions, forming
a part of the potential energy surface.
The PES is the effective potential the nuclei
feel and a part of the Hamiltonian for the
nuclear Schrödinger equation.
Chemical concepts from BO
Electronic structure and nuclear dynamics
(molecular vibration and rotation).
Translational motion is separable exactly.
Potential energy (hyper)surfaces and
curves – effective potentials that nuclei feel.
One PES for each electronic state.
Equilibrium structure, binding energy,
vibrational energy levels, rotational
energy levels, Franck-Condon factors (see
later lectures), nonadiabatic transition.
Chemical concepts from BO
Dynamical degrees of freedom
n electrons + N nuclei: 3(n+N) dynamical
degrees of freedom.
Electronic structure: 3n dynamical DOF.
Nuclear dynamics: 3N dynamical DOF.
Translational DOF: 3.
Rotational DOF: 3 (nonlinear) or 2 (linear).
Vibrational DOF: 3N−6 (nonlinear) or 3N−5
(linear).
Dynamical degrees of freedom
all
3n+3N
Born-Oppenheimer approximation
electronic
3n
nuclear
3N
Exact separation
translational
3
relative
3N−3
Rigid rotor approximation
rotational
3 or 2
vibrational
3N−6 or 3N−5
Summary
The BO is the approximate
separation of variables.
It is one of the most accurate
approximations in chemistry.
This leads to solving (1)
electronic structure with clamped
nuclei and then (2) nuclear
J. Robert Oppenheimer
dynamics with the PES from (1). Public-domain image
This is justified by nuclear mass
>> electron mass.
It is the basis of many chemistry concepts.





![The Politics of Protest [week 3]](http://s2.studylib.net/store/data/005229111_1-9491ac8e8d24cc184a2c9020ba192c97-300x300.png)