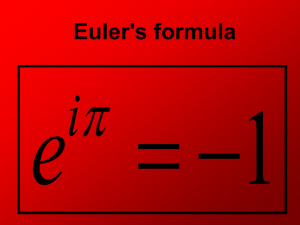
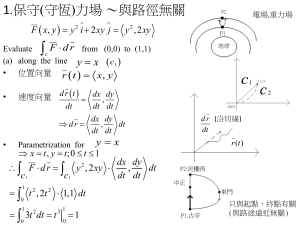
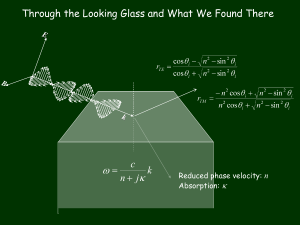
pptx - Weizmann Institute of Science
advertisement

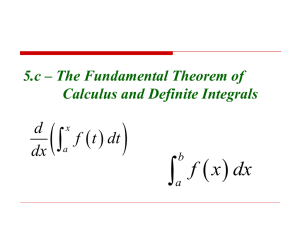
NMR-Primer for Chemists and Biologists Shimon Vega & Yonatan Hovav shimon.vega@weizmann.ac.il and yonatan.hovav@weizmann.ac.il Content November 2013 1. Basic Concepts of Nuclear Spins in a Magnetic Field a. Angular momentum, magnetic moments, magnetization b. Precession of the classical magnetization c. RF irradiation, resonance and the rotating frame d. Concepts of quantum mechanics* e. T1 and T2 Relaxation * Extension depending on students 3. Basic Concept of Pulsed NMR a. The Bloch equations, NMR signals in the laboratory frame b. NMR signals in the rotating frame, quadrature detection c. Manipulating the magnetization and continuous wave spectroscopy d. T1 and T2 measurement, Hahn echo e. Free induction decays and Fourier transform f. FFT, data sampling, spectral width and Nyquist Theorem g. RF pulses, off-resonance effects and composite pulses h. The NMR spectrometer i. Phase cycling j. Digital filtering, pulse programming, the magnet and field inhomogeneity 1 4. The NMR Interactions and 1D spectra a. Chemical shift, isotropic and CSA-interactions b. The vector model and two level system c. Nuclear spin-spin interactions and spectral multiplets d. INEPT and COSY e. Decoupling 5. Two dimensional NMR a. Basic principles b. 2D COSY c. Twisted peaks in 2D NMR, TPPI and STATES d. Examples of 2D experiments e. Nuclear Overhauser Effect and NOESY 6. Solid State NMR Basic principles* * Depending on time left www.rug.nl/zernike/research/groups/phynd/research/spinpolarizedtransport 2 Some books: Modern NMR techniques for Chemistry Research Nuclear Magnetic Resonance spectroscopy Advanced: Spin Dynamics Understanding NMR Spectroscopy Principles of NMR in 1 and 2 Dimensions Principles Magnetic Resonance Solid State NMR Spectroscopy by A.E Derome by R.K Harris by M.H Levitt by James Keeler by R. R. Ernst, G. Bodenhausen A. Wokaun by C. P. Slichter by M Duer Web: 1. http://www.cis.rit.edu/htbooks/nmr/ 2. http://www-keeler.ch.cam.ac.uk/lectures/ 3 Felix Bloch Born (1905-10-23)October 23, 1905 Zürich, Switzerland Died September 10, 1983(1983-09-10) (aged 77) Zürich, Switzerland Citizenship Swiss, American Nationality Swiss Fields Physics Institutions University of California, Berkeley Stanford University Alma mater ETH Zürich and University of Leipzig Doctoral advisor Werner Heisenberg Known for NMR Bloch wall Bloch's Theorem Bloch Function (Wave) Bloch sphere Notable awards Nobel Prize for Physics (1952) Edward Purcell Born (1912-08-30)August 30, 1912 Taylorville, Illinois, USA Died March 7, 1997(199703-07) (aged 84) Cambridge, Massachusetts, USA Nationality United States Fields Physics Institutions Harvard University MIT Alma mater Purdue University Harvard University Doctoral advisor Kenneth Bainbridge Other academic advisors John Van Vleck Doctoral students Nicolaas Bloembergen George Pake George Benedek Charles Pence Slichter Known for Nuclear magnetic resonance (NMR) Smith-Purcell effect 21 cm line Notable awards Nobel Prize for Physics (1952) 4 1920's Physicists have great success with quantum theory Quantum theory was used to explain phenomena where classical mechanics failed. This theory, proposed by Bohr, was particularly useful for the understanding of absorption and emission spectra of atoms. These spectra showed discrete lines which could be accounted for quantitatively by quantum theory. However, this theory still could not explain doublet lines found in high resolution spectra. 1921 Stern and Gerlach carry out atomic and molecular beam experiments The basis of quantum theory was confirmed by the atomic beam experiment. A beam of silver atoms was formed in high vacuum and passed through a magnetic field. 1925 Uhlenbeck and Goudsmit introduce the concept of a spinning electron The idea of a spinning electron with resultant angular momentum gave rise to the magnetic dipole moment. 1926 Schrödinger/Heisenberg formulate quantum mechanics This new branch of quantum physics replaced the old quantum theory. Quantum mechanics was successful for understanding many phenomena but still could not account for doublets in absorption and emmision spectra. 1927 Pauli and Darwin include electron spin in quantum mechanics 1933 Stern and Gerlach measure the effect of nuclear spin Stern and Gerlach increased the sensitivity of their molecular beam apparatus enabling them to detect nuclear magnetic moments. They observed and measured the deflection of a beam of hydrogen molecules. This has no contribution to the magnetic moment from electron orbital angular momentum so any deflection would be due 5 to the nuclear magnetic moment. 1936 Gorter attempts experiments using the resonance property of nuclear spin The Dutch physicist, C.J.Gorter, used the resonance property of nuclear spin in the presence of a magnetic field to study nuclear paramagnetism. Although his experiment was unsuccessful, the results were published and this brought attention to the potential of resonance methods. 1937 Rabi predicts and observes nuclear magnetic resonance During the 1930's, Rabi's laboratory in Columbia University became a leading center for atomic and molecular beam studies. One experiment involved passing a beam of LiCl through a strong and constant magnetic field. A smaller oscillating magnetic field was then applied at right angles to the initial field. When the frequency of the oscillating field approached the Larmor frequency of the nucleus in question, resonance occurred. The absorption of energy was recorded as a dip in the beam intensity as the magnetic current was increased. 1943 Stern awarded the Nobel prize for physics Otto Stern was awarded this prize 'for his contribution to the development of the molecular ray method and discovery of the magnetic momentum of the proton'. 1944 Rabi awarded the Nobel prize for physics Rabi was given this prize for his work on molecular beams, especially the resonance method. 6 1945 Purcell, Torey and Pound observe NMR in a bulk material At Harvard, Purcell, Torey and Pound assembled apparatus designed to detect the transition between nuclear magnetic energy levels using radiofrequency methods. Using about 1kg of parrafin wax, the absorbance was predicted and observed. 1951 Packard and Arnold observe that the chemical shift due to the -OH proton in ethanol varies with temperature. It was later shown that the chemical shift for this proton was also dependent on the solvent. These results were explained by hydrogen bonding. 1952 Bloch and Purcell share the Nobel prize in physics This prize was awarded 'for their development of new methods for nuclear magnetic precision measurements and discoveries in connection therewith'. 1953 A. Overhauser predicts that a small alteration in the electron spin populations would produce a large change in the nuclear spin polarisation. This theory was later to be named the Overhauser effect and is now a very important tool for the determination of complex molecular structure. 1957 P. Lauterbur and C. Holm independently record the first 13C NMR spectra. Despite the low natural abundance of the NMR active isotope 13C, the recorded spectra showed a signal to noise ratio as high as 50. 1961 Shoolery introduces the Varian A-60 high-resolution spectrometer. The Varian A-60 was used to study proton NMR at 60MHz and proved to be the first commercial NMR spectrometer to give highly reproducible results. 7 2. Basic Concepts of Nuclear Spins in a Magnetic Field a. Angular momentum, magnetic moments: magnetization We are dealing with the nuclei of atoms and in particular with their magnetic properties. The nuclei are characterized by their “spin values”. These spins correspond to well-defined angular momenta with values proportional to Planck’s constant: h = 6.6260755x1034 m2kg/sec I with and I 1/ 2 ; 1 ; 3 / 2 ; 2 ; 5 / 2 (from QM) The protons and neutrons (fermions) composing the nuclei determine the nuclear spin value. A nucleus with an odd mass number M has an half-integer spin and a nucleus of an even M has an integer spin. Nuclei with an even number of protons and neutrons have nuclear spin I=0. Each nuclear spin has a magnetic moment proportional to its angular momentum-spin. Proton: g = 5.5856912 +/- 0.0000022 Neutron: g = -3.8260837 +/- 0.0000018 or I For each nucleus the angular momentum vector and the magnetic moment vector are related by its magnetogyric ratio . E .B magnetogyric ratio h .B / B 8 Remember: conservation of angular momentum ! General comments about angular momentum: I I I I r (mv ) r p r 1 / 2mR p m v r moment of inertia v /(2R) [(m / s) /(m) Hz] rp m r2 number of turn per second 2 v / R [(m / s) /(m) sec1 ] number of radians per second General comments about magnetic momentums: q = charge q/L = charge density L = 2r v = velocity i = current A = area A = 2r i e q` q 2 r I r mv r m i A v 2 r 2m 2m i e iA q q q Minimizing energy 1. The torque on the magnetic moment induced by a magnetic field B B 2. Energy of a magnetic moment in a magnetic field E .B Reminder: a b c c a b sin ab B B a x bx a y bz a z by c a , b a y by a z bx a x bz a b a b a b9 y x z z x y 9 Larmor Frequencies in MHz units: L with B B 7.0 Tesla 1T = 10,000G 1H 2H 3H 3 He 6 Li 7 Li 9 Be 10 B 11 B 13 C Proton NMR: 100MHz - 2.3 T 300MHz - 7.0 T Hydrogen Deuterium Tritium Helium Lithium Lithium Beryllium Boron Boron Carbon ½ 1 1/2 1/2 1 3/2 3/2 3 3/2 1/2 500MHz - 11.7 T 800MHz - 18.8 T 300.130 46.073 320.128 228.633 44.167 116.640 42.174 32.246 96.258 75.46 900MHz - 21.1 Tesla (2.1 KHz - 0.5 Gauss) 10 Element/Name Isotope Symbol Nuclear Spin Hydrogen Deuterium Tritium Helium-3 Lithium-6 Lithium-7 Beryllium-9 Boron-10 Boron-11 Carbon-13 Nitrogen-14 Nitrogen-15 Oxygen-17 Fluorine-19 Neon-21 Sodium-23 Magnesium-25 Aluminum-27 Silicon-29 1H 2H or D 3H 3He 6Li 7Li 9Be 10B 11B 13C 14N 15N 17O 19F 21Ne 23Na 25Mg 27Al 29Si Phosphorus-31 31P 33S Sulfur-33 33Cl Chlorine-33 37Cl Chlorine-37 Potassium-39 39K Potassium-41 41K 43K Calcium-43 Scandium-45 45Sc Titanium-47 47Ti Titanium-49 49Ti Vanadium-50 50V Vanadium-51 51V Chromium-53 53Cr Manganese-55 55Mn 57Fe Iron-57 59Co Cobolt-59 61Ni Nickel-61 63Cu Copper-63 65Cu Copper-65 67Zn Zinc-67 1/2 1 1/2 -1/2 1 3/2 -3/2 3 3/2 1/2 1 -1/2 -5/2 1/2 -3/2 3/2 -5/2 5/2 -1/2 1/2 3/2 3/2 3/2 3/2 3/2 -7/2 7/2 -5/2 -7/2 6 7/2 -3/2 5/2 1/2 7/2 -3/2 3/2 3/2 5/2 Sensitivity vs. 1H 1.000000 1.44 e-6 0.000628 0.270175 0.013825 0.00386 0.132281 0.000175 0.000998 3.84E-06 1.07E-05 0.829825 6.3E-06 0.092105 0.00027 0.205263 0.000367 0.06614 1.71E-05 0.003544 0.000661 0.000472 5.75E-06 9.25E-06 0.3 0.00015 0.00021 0.00013 0.37895 8.6E-05 0.174386 7.37E-07 0.275439 4.21E-05 0.064035 0.035263 0.000117 Frequency (MHz) Receptivity : (natur.abund.-%) x x I(I+1) 1/2 Tesla 5 x10-5 2.35 7.05 9.40 11.75 18.80 21.15 1 3/5 5/2 7/2 MHz 2.1 x10-3 100 300 400 500 800 900 11 2b. The classical precession of the magnetization B Suppose we apply a magnetic field on our magnetization: as a result a torque tries to rotate the direction of the angular momentum. I A torque ( r F ) perpendicular to an angular momentum causes a precession motion: Example: top view: From http://hyperphysics.phy-astr.gsu.edu Remember the motion of a top: (gravitation + top) 12 The precession of the magnetization around the magnetic field direction is independent of the orientation of F ma dp / dt B (in analogy with ) F z I B 0 B The Larmor frequency y dI ; dt B x I I ; I x , y B sin( / 2) I dI B I B 0 I dt Ix I sin cos (0 I sin ) sin 0 I y d d I y I sin sin (0 I sin ) cos 0 I x dt dt 0 0 Iz I cos Ix I x cos L t I y sin L t I y (t ) I x sin L t I y cos L t I I z z 13 2c. RF irradiation, resonance and the rotating frame The equation of motion for the magnetization in an external magnetic field Let d (t ) (t ) B L (t ) dt y z z y x d y (t ) x z z x dt y x z x y z L ; B (t ) y x Let us now consider a special time-dependent magnetic field: 1 cost B B0 z B1 (cos t x sin t y ) 1 sin t 0 B0 0 | B1 | 1 z B0 () How does magnetic moment respond? x B1 y Laboratory frame 14 To follow the response of the magnetization let us rotate the coordinate system: xRoF (t ) cost sin t 0 x (t ) x (t ) cost y (t ) sin t RoF y (t ) sin t cost 0 y (t ) x (t ) sin t y (t ) cost RoF (t ) (t ) 0 0 1 ( t ) z z z Then we get the equation of motion: xRoF (t ) (d / dt x (t )) cost x sin t (d / dt y (t )) sin t y cost d RoF y (t ) (d / dt x (t )) sin t x cost (d / dt y (t )) cost y sin t dt RoF (d / dt z (t )) z (t ) and insertion of the original equation of motion: we get x (t ) 0 y (t ) (1 sin t ) z (t ) d y (t ) 0 x (t ) (1 cost ) z (t ) dt ( cost ) (t ) ( sin t ) (t ) ( t ) y 1 x z 1 xRoF (t ) (0 y 1 z sin t ) cost x sin t (0 x 1 z cost ) sin t y cost d RoF y (t ) (0 y 1 z sin t ) sin t x cost (0 x 1 z cost ) cost y sin t dt RoF (1 y cost 1 x sin t ) z (t ) Thus the equation of motion in the rotating frame becomes: xRoF (0 ) yRoF d RoF RoF RoF y (0 ) x 1 z dt RoF RoF 1 y z 15 Thus in the rotating frame the magnetic field becomes time-independent while the z-magnetic field component is reduced by the frequency of rotation zRoF RoF 1 xRoF yRoF rotating frame (0 ) ,there 0 is only an x-components to the field. On-resonance, when In such a case the magnetization performs a precession around the x-direction with a rotation frequency .1 How to generate this B1 RF irradiation field in the laboratory frame: B0 B1 (t ) B1 cost I (t ) I1 cost 21 cost x y z Ignore because it is off-resonance! 1 (cost x sin t y) 1 (cost x sin t y ) x Top view y x 16 Bird cage National High Magnetic Field Laboratory Doty Scientific u-of-o-nmr-facility.blogspot.com/2008/03/prob... in: Thus the magnetic field in the laboratory frame : LAB 0 z 1 cos(t ) x 1 sin(t ) y Becomes in the rotating frame: RoF z 1 cos x 1 sin y out: RoF , x (t ) In NMR we measure the magnetization in the rotating frame: RoF , y (t ) Although the signal detection in the laboratory frame is along the direction of the coil: LABx (t ) xRoF (t ) cost yRoF (t ) sin t A sample with an overall M (t ) the S/N voltage at the coil is: S/N f 1/ 2 Vs 1 Q M 0 kT 2 f =noise of apparatus =filling factor =frequency =band width Q =quality factor Vs =sample volume 17