Chapter 10 Spinodal Decomposition in Binary Polymer Blends
advertisement

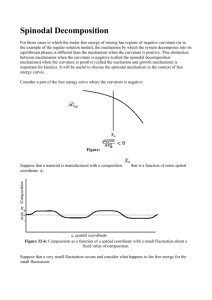
Chapter 10 Spinodal Decomposition in Binary Polymer Blends M a t E 4 5 4 | A p r i l 2 2 nd, 2 0 1 4 MOHAMMED ALZAYER EDWARD BRUNS XIAOLIN BI Outline • Introduction • Theory: Ideal Solution Model Regular Solution Model Flory-Huggins Theory The Cahn-Hilliard Model Fox Equation • Binary Systems Exhibiting SD: PMMA/ PαMSAN PMMA/SAN PEH/PEB PMMA/PLLA Incommensurate Films Composition-dependent heat conductivity systems • Conclusion Introduction [1] • Lower Boundary: thermally induced mixing • Upper Boundary: thermally induced demixing • Maximum: Highest T for mixing (UCST) • Minimum: Lowest T for demixing (LCST) [1] Simmons, D. S. (2009). Phase and conformational behavior of lcst-driven stimuli responsive polymers. (Doctoral dissertation, University of Texas) Introduction [1] • Other Behaviors: LCST is the most common There are others: (a), UCST but.. only (b), LCST and UCST curves with multiple extrema (c and d). merged LCST and UCST (e), closed immiscibility loops (f), and combinations of LCST, UCST, and closed immiscibility loop behavior (g and h). Curves may represent either spinodal or binodal curves. [1] Simmons, D. S. (2009). Phase and conformational behavior of lcst-driven stimuli responsive polymers. (Doctoral dissertation, University of Texas) Introduction To be immiscible, i.e. spinodal decomposed: • Criteria #1: 2 or more chemically different polymers in in a shared volume • Criteria #2: Phase separation between the polymers, macrosized regions of similar-chemical polymer, or single polymer rich regions dispersed throughout homogenous mixture The Ideal Solution Model [2] • Obeys Raoult’s: 1 (solvent) while 2 (solute). a is the activity of the component, X is the mole fraction, Pi is the vapor pressure of the solvent before mixing, and Pf is the vapor pressure of the solvent after mixing • Enthalpy and entropy of mixing: [2] Murat, S. (2010). Physical chemistry of polymers: Thermodynamics of solutions of high polymers. (Doctoral dissertation, Hacettepe University, Ankara, Turkey) The Ideal Solution Model [2] • Why does it fail to describe polymer blends? A solution with a very small solute weight fraction as well as a small mole fraction (𝑋2 ) can hardly deviate from the ideality. Polymer solutions consist of polymeric solutes with high molecular weights and mole fractions (99%!) [2] Murat, S. (2010). Physical chemistry of polymers: Thermodynamics of solutions of high polymers. (Doctoral dissertation, Hacettepe University, Ankara, Turkey) The Regular Solution Model [3][4] • How does it describe SD? Formation of uni-polymer rich regions or phase separation of polymers from a seemingly uniform matrix/mixture. • Why does it happen? SD occurs as a result of compositions lowering blend’s Gibbs free energy [3] Martin, B. (2011). Phase transformations: Nucleation and spinodal decomposition. MIT. [4] Zang, L. Spinodal Decomposition: Part 1: General Description and Practical Implications. The University of Utah. The Regular Solution Model [3][4] • Notes on the model: Points where 𝝏𝟐 𝑮/𝝏𝒙𝟐 = 0 called spinodes (inflection points). Spontaneous phase separation faces no thermodynamic barrier. i.e. controlled solely by diffusion. [3] Martin, B. (2011). Phase transformations: Nucleation and spinodal decomposition. MIT. [4] Zang, L. Spinodal Decomposition: Part 1: General Description and Practical Implications. The University of Utah. Flory-Huggins Theory [5][6] • considers a low MW solvent and a high MW polymer in a lattice: Where xi is molar fraction of the component, Z is coordination number (nearest # of neighbors in lattice), N is total number of lattice sites, ∆𝑤 is ~ energy of formation, and 𝜑 is fraction of lattice sites occupied. • And the Flory Parameter is: [5] Frank, C. (2001). Flory-huggins model for polymer solutions. Stanford University. [6] Andersson, C. (2008). Flory-huggins theory applied in atmospheric aerosol modelling. (Master's thesis, Stockholm University) Flory-Huggins Theory [5][6] • Why is χ commonly used? It is independent of concentration It gives a better approximation of a: Where 𝑎𝑤 is activity of water, ∅ is volume fraction of polymer, and r is chain segment number (polymer volume to water volume ratio). [5] Frank, C. (2001). Flory-huggins model for polymer solutions. Stanford University. [6] Andersson, C. (2008). Flory-huggins theory applied in atmospheric aerosol modelling. (Master's thesis, Stockholm University) The Cahn-Hilliard Model [7] • Why another model? 1) Regular & ideal too simple to model real cases 2) It considers chemical kinetics The difference in concentration is given by where c is the concentration , 𝑅𝑚 is the amplification factor of the fastest growing wavelength, t is time, 𝛽 is wavenumber, and 𝛽𝑚 is the dominate wavenumber during system decomposition. [7] Bukusoglu, E., Pal, S. K., De Pablo, J. J., & Abbott, N. L. (2014). Colloid-in-liquid crystal gels formed via spinodal decomposition. Soft Matter, (10), 1602-1610. The Cahn-Hilliard Model [7] • Why another model? Dynamics of the SD modeled as a function of the depth of the thermal quench (∆𝑇): [7] Bukusoglu, E., Pal, S. K., De Pablo, J. J., & Abbott, N. L. (2014). Colloid-in-liquid crystal gels formed via spinodal decomposition. Soft Matter, (10), 1602-1610. Fox Equation [8] • What is it? Fox Equations among others utilized to predict Tg [8] Madbouly, S. A. (2014). Mat E 454: Polymer composites and processing (Lectures). Iowa State University. Fox Equation [8] • What is it? Fox Equations among others utilized to predict Tg [8] Madbouly, S. A. (2014). Mat E 454: Polymer composites and processing (Lectures). Iowa State University. PMMA/ PαMSAN [9] • Preparation: 1) Drying at r.t., 3 days, cast solution in Petri. 2) Further dried by vacuum for 3 days at 90 °C. 3) Meltpressing on a hot chamber, at constant T. 4) After annealing, thin film obtained, t=40 mm. tetrahydrofuran PMMA PαMSAN (31 wt% acrylonitrile) 𝑀𝑊 , PDI 14000 g/mol, 2.1 96500 g/mol, 2.26 Techniques: Optical, DSC (10 °C/min), Timeresoloved light scattering (632.8 nm He-Ne) [9] Madbouly, S. A., & Ougizawa, T. (2004). Spinodal decomposition in binary blend of PMMA/ PαMSAN: Analysis of early and late stage demixing. Macromolecular Chemistry and Physics, 205(7), 979–986. PMMA/ PαMSAN [9] • Some observations: Near critical composition 75:25. 1-phase (150 °C) to 2 phases (180 °C). Connectivity not clear until 20 mins. Annealing time increased, contrast of 2-phase increased. Late stage of SD (50 mins), co-continuity lost result of coarsening. “fragmented particles.” 10 min 20 min 50 min [9] Madbouly, S. A., & Ougizawa, T. (2004). Spinodal decomposition in binary blend of PMMA/ PαMSAN: Analysis of early and late stage demixing. Macromolecular Chemistry and Physics, 205(7), 979–986. PMMA/ PαMSAN [9] • Notes: LCST, miscible at a limited T range, miscible at entire w range (1 common 𝑇𝑔 , Fox), χ increases a lot with T (slope shift from negative to a small positive) agrees with LCST. [9] Madbouly, S. A., & Ougizawa, T. (2004). Spinodal decomposition in binary blend of PMMA/ PαMSAN: Analysis of early and late stage demixing. Macromolecular Chemistry and Physics, 205(7), 979–986. PMMA/SAN [10] • Why add nanoparticles? Ability to control morphology, improve electrical properties, change phase separation T and phase diagram. Behavior becomes more complicated. One of the polymers absorbs the other, changing the thermodynamics. PMMA SAN 𝑀𝑊 , PDI 15.9 × 104 , 1.64 14.1 × 104 , 2.08 𝑇𝑔 96 °𝐶 105 °𝐶 [10] Gao, J., Huang, C., Wang, N., Yu, W., & Zhou, C. (2012). Phase separation of PMMA/SAN blends in the presence of silica nanoparticles. Polymer, 53(8), 1772–1782. PMMA/SAN [10] • Ajji and Choplin’s Equation to get Ts and Tb: [10] Gao, J., Huang, C., Wang, N., Yu, W., & Zhou, C. (2012). Phase separation of PMMA/SAN blends in the presence of silica nanoparticles. Polymer, 53(8), 1772–1782. PMMA/SAN [10] • Effect of size: The bigger the particle, the lower the Tb. Micron sized hardly have an effect (Tb ~ Tb pure). Tb increases with more particles added. SiO2 content 3% 3% 3% - 1% 5% SiO2 diameter (nm) 12 30 1000 - 30 30 Tb (°C) 172.2 171.8 169 167 171.3 174.5 [10] Gao, J., Huang, C., Wang, N., Yu, W., & Zhou, C. (2012). Phase separation of PMMA/SAN blends in the presence of silica nanoparticles. Polymer, 53(8), 1772–1782. PEH/PEB [11] 𝑀𝑊 Contains Thickness and shape PEH PEB 110000g/mol 70000g/mol 2 mol% hexane 15 mol% butane 0.5 mm dog bone • Preperation: Heat treatments (separation at 130 oC), quenched into liquid nitrogen causing fracture, etched by 1% potassium permanganate in a mixture of sulfuric acid and orthophosphoric acid for contrast. [11] Yang, L., Yanhua, N., Wang, H., & Wang, Z. (2009). Effects of spinodal decomposition on mechanical properties of a polyolefin blend from high to low strain rates. Polymer, 50(13), 2990–2998. PEH/PEB [11] • High strain rate (0.01s-1): interfacial relaxation between phase domains cannot be detected. • Low strain rate (0.001s-1): drop of tensile properties with separation when Tc is low. The effect disappears at high Tc. [11] Yang, L., Yanhua, N., Wang, H., & Wang, Z. (2009). Effects of spinodal decomposition on mechanical properties of a polyolefin blend from high to low strain rates. Polymer, 50(13), 2990–2998. PMMA/PLLA [12] • In the figure: “Tapping-mode AFM images of monolayers mixtures (25/75,50/50, and 75/25 weight fraction) deposited on mica at surface pressure 1, 5, 10, and 12 mN/m. Fibrils at surface pressure higher than 10 mN/m are crystallized PLLA lamella.” [12] Sato, G., Nishitsuji, S., & Kumaki, J. (2013). Two-dimensional phase separation of a poly(methyl methacrylate)/poly(l-lactide) mixed langmuir monolayer via a spinodal decomposition mechanism.The Journal of Physical Chemistry, 117(30), 9067–9072. PMMA/PLLA [12] • 2D: Analogous to 3D’s nucleation and growth • Spinodal ring: FFT shows doughnut like pattern in inserts. Phase-separated structures possess concentration fluctuation with a specific λ: • Early Stage: wavelength of dominant mode is independent of t, whereas the concentration fluctuations, Δϕ(t), grow with time [12] Sato, G., Nishitsuji, S., & Kumaki, J. (2013). Two-dimensional phase separation of a poly(methyl methacrylate)/poly(l-lactide) mixed langmuir monolayer via a spinodal decomposition mechanism.The Journal of Physical Chemistry, 117(30), 9067–9072. PMMA/PLLA [12] • Intermediate stage: described by both λ and Δϕ(t) growing with time. • Final stage: λ increases with time, while Δϕ(t) already saturates to its equilibrium value. [12] Sato, G., Nishitsuji, S., & Kumaki, J. (2013). Two-dimensional phase separation of a poly(methyl methacrylate)/poly(l-lactide) mixed langmuir monolayer via a spinodal decomposition mechanism.The Journal of Physical Chemistry, 117(30), 9067–9072. Incommensurate Films [13] • Top: 2.5 µm of film surface at 160 min, majority perpendicular lamellar morphology (PS dark, PMMA light). • Bottom: SCFT calculation of mixed morphology intermediate state. [13] Peters, R. D., Pawel, S., Matsen, M. W., & Dalnoki-Veress, K. (2013). Morphology induced spinodal decomposition at the surface of symmetric diblock copolymer films. ACS Macro Letters, 2(5), 441–445. Composition-dependent Heat Conductivity Systems [14] • Quench conditions: structure resulting from SD varies with quench condition. • Example: in the figure, the left wall is quenched, while the right wall is insulated [14] Molin, D., & Mauri, R. (2008). Spinodal decomposition of binary mixtures with composition-dependent heat conductivities. Chemical Engineering Science, 63(9), 2402–2407. Composition-dependent Heat Conductivity Systems [14] λ stands for heat conductivity ratio, while â is a characteristic length and D is a mass diffusivity parameter. Overall, the 105 â2/D translates between 1-10 seconds. NLE, the lewis number, stands for the ratio of thermal to mass diffusivity. [14] Molin, D., & Mauri, R. (2008). Spinodal decomposition of binary mixtures with composition-dependent heat conductivities. Chemical Engineering Science, 63(9), 2402–2407. Conclusion • From this presentation: we can conclude that spinodal decomposition is important in polymers science. • We can take it further: improve the miscibility of blends by introducing a third polymer (compatibilizar). • It’s beyond this chapter: no longer binary, but rather ternary. • Why ternary? High concentration of third polymer is needed.