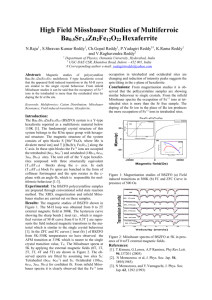
Mossbauer Spectroscopy of Europium
advertisement

Mössbauer Spectroscopy: EuropiumContaining Compounds and High Pressure studies Corey Thompson Technique Presentation 03/21/2011 Mössbauer Effect Mössbauer Effect - was discovered by R.L. Mössbauer during his Ph.D work in 1957 (Nobel Prize in 1961). • It involves the emission and absorption of gamma rays in atoms in solids and forms the basis of mössbauer spectroscopy. Mössbauer Spectroscopy Mössbauer Spectroscopy - probes minute changes in the energy levels of an atomic nucleus in response to its chemical environment. Can provide the following information: • indication of purity; • characterize the oxidation state of the atom of interest; • prove whether one compound has a different structure from another of the same composition; • indicate whether two or more nuclei in a polynuclear compound are in equivalent environments; • and give information on the magnetic ordering of the compound. Mössbauer Spectrometer Source Collimator Doppler Effect Sample Absorbance Detector -Ve 0 Velocity +Ve Suitable Sources Most important criteria: • Radioactive isotope emits gamma ray of less than 150keV (57Fe- 14.4keV or 151Eu – 21.54keV) • Gamma ray well separated in energy from other photons • Half-life of precursor to be long (57Co ~ 270days or 151Sm ~ 90 yrs.) • line-width be small Sample Collection and Prep. • Sample is powdered and spread across a sample holder (use of sucrose or graphite helps spread sample evenly across holder) • Sample is then held in place with something thin and non-absorbant to γrays such as cellophane or kapton tape. • Sample amount matters and if chemical composition is known there is a equation that can compute how much sample is needed (use too little then some γ-rays will not encounter an Eu atom; use too much can affect area, intensity, width and etc.) Mössbauer Spectrum 3 Types of Nuclear interactions that are observable: • Isomer (chemical, )shifts • Quadrupole splitting • Magnetic (hyperfine) splitting Isomer (Chemical, ) Shifts eE 0 sE γ aE γ aE γ > sEγ gE 0 Source Absorber Absorbance Isomer shifts - results from the electrostatic interaction between the charge distribution of the nucleus and those electrons which have a finite probability of being found around the nucleus(only s electrons have a finite probability of overlapping the nuclear density; can be influenced by p,d electrons by screening the s density from nuclear charge; think SLATER’S RULES and Zeff= Z-S) • Does not lead to splitting of energy levels but results in a slight shift of mössbauer energy levels in a compound relative to the source -Ve 0 Velocity = aEγ - sEγ +Ve Quadrupole Splitting Quadrupole splitting – the interaction of non-spherical or cubic extranuclear electric fields with the nuclear charge density resulting in splitting of the nuclear energy levels. • For half-integral nuclear spins, the quadrupole interaction results in I + ½ levels for spin I. For integral nuclear spins, the degeneracy of the nuclear levels may be completely removed by quadruple interaction to give 2I + 1 levels. Ex: Fe 3/2 1/2 3/2 Q.S. 1/2 Absorbance I mI Q.S. 1/2 Quadrupole Splitting C.S. (from source) -1 0 +1 Velocity (mm s-1) Magnetic (hyperfine) splitting Magnetic splitting – is a result of the interaction between the nucleus and any surrounding magnetic field. The nucleus spin I, splits into 2I+1 sublevels. The selection rules mI= 0, 1 give rise to a symmetric 6-line spectrum. mI I 6 +3/2 3 5 eE a +1/2 3/2 2 4 1 1/2 -1/2 -3/2 -1/2 gE a Magnetic Splitting +1/2 Absorbance Ex: Fe 3 4 2 5 6 1 -Ve 0 Velocity (mm s-1) +Ve Data Collection and Processing • Techniques for processing Mössbauer data are complex and variable. • There is many software out there to analyze data such as Mosswinn. • Software uses a variety of different models to generate model spectra to compare to the measured spectra. • Three different lines shapes are commonly employed in modeling of spectra but most used are Lorentzian and Voigt. Eu-Containing Compounds • Source – 151Sm ~ 90yrs (SmF3); reference to EuF3 • 151Eu – 21.54 keV Mössbauer transition 153Eu – has 3 Mössbauer transitions (83.37, 97.43, and 103.18 keV); complex nuclear levels and source have short half-lives (153Sm ~ 46.7hrs) Eu-Containing Compounds: Mossbauer Spectroscopy • Velocity range -> -30 to +30 mm/s • Eu2+ Isomer shifts are in the range of -13 to -8 mm/s • Eu 3+ Isomer shifts are in the range of 0 to 5 mm/s • Usually see isomer shifts and magnetic hyperfine interactions (line width is too big to see quadrupole splitting) Eu-Containing Compounds: Quadrupole Interaction Eu-Containing Compounds: Magnetic Interaction MS of EuM2P2, M = Co and Ni EuNi2P2 no magnetic ordering Intermediate Valence EuCo2P2 AFM 67K Valence is 2+ High Pressure: EuM2Ge2,M = Ni and Pd Eu2+ Intermediate Valence Eu3+ Eu2+ Intermediate Valence Eu3+ Conclusion • Mössbauer spectroscopy is a technique that gives you information about the nucleus chemical environment. • Three types of interactions are involved: isomer shifts, quadrupole splitting, and magnetic splitting. • Factors that govern the shifts are the chemical environment (ligands), the nuclear charge (shielding of s density due to p,d electrons), and etc. • Can determine oxidation states, high/low spin compounds, determine magnetic ordering, and indicate whether you have two different nuclei in a polynuclear compound.