Microscopies" PPT - The Parker Lab at UCI
advertisement

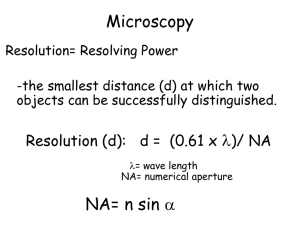
Functional cellular imaging by light microscopy MICROSCOPIES Why use Light? Good (enough) resolution: Spatial – classically a few hundred nanometers; now tens of nm Temporal - <millisecond Compatible with live cells, tissues, organisms Many probes available for imaging: Fluorescent antibodies, GFP, indicators for Ca2+ membrane potential, etc. Relatively inexpensive and simple (vs. E.M., PET, FMRI etc) Three decades of building microscope systems 1977 2008 On the importance of looking “You can observe a lot just by watching.” Yogi Berra Former Yankee Catcher and Great American Sage And sometimes you can see beautiful things! Chaotic and spiral calcium waves in Xenopus oocyte: Jim Lechleiter, U. Texas Major types of light microscopy; 1. Transmitted (reflected) light. Poor contrast; paucity of specific labels/functional probes; poor depth resolution. 2. Fluorescence. High contrast (black background); numerous fluorescent dyes, proteins and functional probes; permits 3D imaging (confocal, 2-photon) A fluorescence microscope But – conventional fluorescence imaging provides little depth discrimination, so images are terrible because of out-of-focus fluorescence e.g. Pollen grain imaged by conventional epifluorescence microscopy One solution – Confocal microscopy Out-of-focus light is rejected by blocking it with a pinhole aperture Confocal sections through pollen grain at 1 um intervals 3-D reconstruction of pollen grain Another way to avoid out-of-focus fluorescence and achieve 3D imaging – Two-photon microscopy Especially good for looking deep into tissues (e.g. brain) without damaging cells Practical theory of 2-photon microscopy 1. Near simultaneous absorption of the energy of two infrared photons results in excitation of a fluorochrome that would normally be excited by a single photon of twice the energy. 2. The probability of excitation depends on the square of the infrared intensity and decreases rapidly with distance from the focal volume. Advantages of 2-photon microscopy 1. 2. 3. 4. 5. Increased penetration of infrared light allows deeper imaging. No out-of-focus fluorescence. Photo-damage and bleaching are confined to diffractionlimited spot. Multiple fluorochrome excitation allows simultaneous, diffraction-limited, co-localization. Imaging of UV-excited compounds with conventional optics. Two-photon imaging of exocytosis in pancreatic acinar cells Exocytic events evoked by addition of acetylcholine Single-cell imaging in intact lymph node 100 m PF AL DC TZ M C 25 m EL 5 m 15 m Miller et al., 2002. Science 296: 1869-1873 Another solution – Total Internal Reflection (TIRF) Microscopy Excite fluorescence in only a very thin layer right next to a coverglass Good for looking at things happening in or very near the plasma membrane of a cell Total internal reflection microscopy air glass glass Total internal reflection microscopy Evanescent wave air glass Through-the-lens total internal reflection fluorescence microscopy (TIRFM) © Molecular Expressions Microscopy Primer Cultured cells expressing GFP-tagged membrane protein imaged by conventional epifluorescence The same cells viewed by TIRFM TIRFM imaging of single-channel Ca2+ fluorescence signals (SCCaFTs): Ca2+ entry through plasma membrane channels expressed in Xenopus oocytes Imaging single channel events with high time resolution: SCCaFTs recorded at 500 frames s-1 The diffraction limit © Molecular Expressions Microscopy Primer Sidling around the diffraction limit The position of a single point source (e.g. a fluorescent molecule) can be localized with much higher precision, limited only by the number of photons that can be collected. What we then need is to have only sparse sources at any given time, so as to avoid unresolved overlap Photoactivation Localization Microscopy (PALM) (Betzig et al., Science 2006) • Express protein of interest tagged with a photoactivatable fluorescent protein (eg.g. EOS) in cell • Stochastically photoactivate a low density of molecules per frame and localize using Gaussian function Excitation laser 532 nm Activating laser 405 nm Non-fluorescent state Fluorescence emission active state Bleached state Repeat thousands of times Photoactivation Localization Microscopy (PALM) • Express protein of interest tagged with a photoactivatable fluorescent protein (eg.g. EOS) in cell • Stochastically photoactivate a low density of molecules per frame and localize using Gaussian function Imaging actin tagged with EosFP (photoactivatable protein) Eos-actin TIRF Eos-actin PALM Fibroblasts expressing DsRed “Greening” of DsRed “greening” results from enhancement of green fluorescence and reduction of red fluorescence Clustered T cells after activation Evanescent field excites Ca2+-dependent fluorescence only in a thin layer next to cell membrane Ca2+ indicator (fluo-4) in cytosol