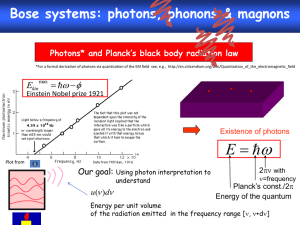
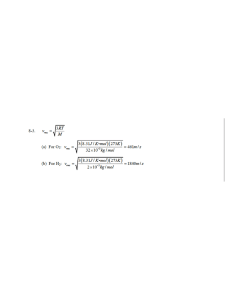
multiple choice
advertisement
Lecture 1 Copyright ©The McGraw-Hill Companies, Inc. Permission required for reproduction or display. PRACTICE TEST MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE The active agent in common household bleach is Sodium hypochlorite. Calculate the pH of 0.15M NaOCl solution (Ka HOCl = 3.0 x 10-8 ) MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE MULTIPLE CHOICE Which of the following is false? A. Visible photons carry more energy than infrared photons. B. Microwave photons carry more energy than infrared photons. C. Ultraviolet photons carry more energy than visible photons. D. X-ray photons carry more energy than microwave photons. MULTIPLE CHOICE A D2 arc lamp A. is the most widely used source of visible photons. B. is the most widely used source of ultraviolet photons. C. is the most widely used infrared source. D. has a continuous output spanning the ultraviolet and visible ranges. MULTIPLE CHOICE Double beam spectrometers A. employ an optical chopper or beam director to alternate the source beam between the reference and sample compartments. B. usually employ a motor drive to move the dispersive element in the monochromator. C. automatically correct for changes in source output with wavelength. D. All of the above. MULTIPLE CHOICE Which of the following is true? A. If an analysis is accurate, it must also be precise. B. A precise analytical measurement will always have a small relative standard deviation. C. If an analysis is reproducible, it will be accurate. D. None of the above. MULTIPLE CHOICE Sketch a calibration curve generated using the method of standard additions. Name one advantage offered by the method of standard addition over a conventional analysis. SHORT ANSWERS In a ultraviolet-visible spectrometer, why is the sample placed after the monochromator? SHORT ANSWERS Compute the energy in joules of a photon whose wavelength is 321 nm SHORT ANSWERS List five of the six numerical criteria for selecting analytical methods. SHORT ANSWERS How does absorption and emission of radiation by atoms differ from that absorbed and emitted by molecules. Comment on the absorption spectrum of an atomic vapor versus a molecular vapor. SHORT ANSWERS Compare and contrast continuum and lines sources. Give at least one example of each. MULTIPLE CHOICE What is a laser? What are the advantages of using lasers as spectroscoptic sources. MULTIPLE CHOICE How many fundamental modes of vibration are predicted for methane (CH4)? Diagram one vibrational mode that you would expect to not be IR active. MULTIPLE CHOICE Sketch a diagram of a single beam UV-VIS spectrophotometer. Label the parts. Describe the basic difference between atomic emission and atomic absorption spectroscopy. Carbon monoxide (CO) can be determined at trace levels using IR absorption spectrophotometry. Using a 100.0-cm pathlength gas cell, a standard containing 10.0 ppm CO gave an absorbance of 0.050 at 2170 cm-1. Calculate the concentration (ppm) of a gas sample. PROBLEM SOLVING Molar absorptivity data for the cobalt and nickel complexes with 2,3-quinoxalinedithiol are: , nm 510 nm 5520 36400 656 nm 17500 1240 (Ni) (Co) A 0.425 g sample was dissolved and diluted to 50 mL. A 25.0 mL aliquot was treated to eliminate interferences; after addition of 2,3-quinaxalinedithiol, the volume was adjusted to 50.0 mL. This solution has absorbance of 0.446 at 510 nm and 0.326 at 656nm. Calculate the ppm of Ni and Co in the sample. Li was determined by atomic emission with the method of standard addition. Prepare a standard addition graph to find the concentration of Li. The standard contained 1.62 g Li/mL. Unknown, mL Standard, mL Final Volume, mL Emission intensity 10.0 0.00 100.0 309 10.0 5.00 100.0 452 10.0 10.00 100.0 600 10.0 15.00 100.0 765 10.0 20.00 100.0 906 SAMPLE PROBLEM