10. - iannonechem.com
advertisement

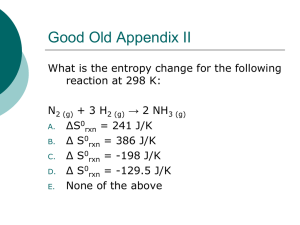
Enthalpy and Entropy Lesson # 10 There are two factors that determine the relative amount of reactants and products at equilibrium. 1. Reactions tend to proceed toward minimum enthalpy. 2. Reactions tend to go toward maximum entropy Enthalpy is PE – In an Exothermic Reaction A + B = C + energy Products are Favoured The low side (side with the energy) is always favoured- minimum Enthalpy is PE – In an Endothermic Reaction A + energy = B + Reactants are Favoured The low side (side with the energy) is always favoured- minimum C Entropy is randomness or a measure of how spread out, or broken up the system is. Less entropy (s) (l) (aq) (g) More Entropy Less Entropy More Entropy H2O(l) H2O(g) H2O(s) Watch Simulation H2O(l) NaCl(s) NaCl(aq) N2O4(g) 2NO2(g) P6O9(g) 3P2O3(g) glass broken glass clean room messy room You can tell the yeild of a reaction with enthalpy and entropy √ max entropy √ min enthalpy √ ⇌ ⇌ √ √ equlibrium √ products favoured If they are both on one side that side is favoured If they are on different sides it’s an equilibrium Are the products or Reactants favoured? 0 gases 1. Zn(s) + 2HCl(aq) ? ↔ 1 gas H2(g) + ZnCl2(aq) +E ΔH = -252 KJ For entropy- only look at phase symbols- mainly gases √ max entropy √ min enthalpy Large Keq Products are favoured Which Way? Goes to Completion Right or left? High yield Reactants ⇌ Products Are the Products or Reactants favoured? 2. +E N2O4(g) ? ↔ 1 gas ΔH = +20 KJ 2NO2(g) 2 gases √ max entropy √ min enthalpy Which Way? Keq is about 1 Right or left? Equilibrium reactants ⇌ Products Are the Products or Reactants favoured? 3. 3C(s) + 3H2(g) + 45 KJ ? ↔ C3H6(g) √ max entropy √ min enthalpy Keq is small Reactants are favoured Low yield Reaction does not occur! reactants ⇌ products 4. Describe the change in entropy and enthalpy for an endothermic equilibrium system. Enthalpy must increase Entropy must increase I’m Entropy Maximum entropy Must be on the opposite side Minimum enthalpy 5. Describe the change in entropy and enthalpy for an exothermic equilibrium system. I’m Entropy Maximum entropy Enthalpy is decreasing Minimum enthalpy Entropy is decreasing 6. Describe entropy and enthalpy as increasing or decreasing. Zn(s) + 2HCl(aq) → H2(g) + ZnCl2(aq) ΔH = -252 KJ √ max entropy √ min enthalpy Enthalpy decreases Entropy increases 7. Describe entropy and enthalpy as increasing or decreasing. 3C(s) + 3H2(g) + 45 KJ → √ min enthalpy √ max entropy Enthalpy increases Entropy decreases C3H6(g) Equilibrium is a compromise between the tendencies toward minimum enthalpy and maximum entropy.