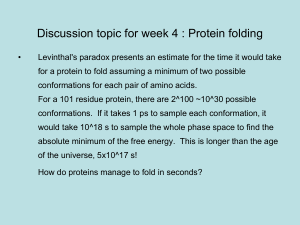
Keq - St John Brebeuf
advertisement

Equilibrium Calculations Lesson 5 How can we describe an equilibrium system mathematically? reactants ⇌ products products Keq = = 3.0 reactants The Keq is the equilibrium constant- a number that does not change. Providing the temperature is kept constant. Equilibrium Calculations An equilibrium system, at any given temperature, can be described by an equilibrium expression and equilibrium constant. aA + bB ⇌ cC + dD Products Keq = Reactants [C]c[D]d Keq = [A]a[B]b Equilibrium Constant- a number Expression- mathematical equation (aq) and (g) are included in the expression! (l) (pure liquids, meaning it is the only one in the equation) and (s) are not because they have constant concentration! Only changes to (aq) and (g) reactants or products cause the equilibrium to shift. (s) and (l) do not! Solids (s) and pure liquids (l) have constant concentrations. Solids & Liquids have fixed densities, cannot be compressed, so their molar concentrations are constant (s) And (l) concentrations are already included in Keq value so we don’t include them in the equation. CaCO3(s) + 2H+(aq) + 2Cl-(aq) ⇌ Ca2+(aq) + Cl2 (g) + CO2(g) + H2O(l) no shift right left left right left no shift 1. At equilibrium at 25oC, [SO3] = 0.200 M. [H2O] = 0.480 M, and [H2SO4] = 24 M. Calculate the Keq. No ICE SO3(g) ⇌ + H2O(g) 1 Keq = H2SO4(l) don’t count (l)! Use 1 [SO3] [H2O] = 1 (0.200)(0.480) = 10.4 The Keq has no units but concentration units that go in the expression must be M! 2. 0.500 mole PCl5, 0.40 mole H2O, 0.200 mole HCl, and 0.400 mole POCl3 are found in a 2.0 L container at equilibrium at 125 oC. Calculate the Keq. No ICE PCl5(s) + H2O(g) ⇌ 2HCl(g) + POCl3(g) = 0.200 moles 2.0 L [HCl] [POCl3] = 0.400 moles = 0.10 M = 0.20 M Keq = 2.0 L [H2O] = 0.40 moles 2.0 L [HCl]2[POCl3] [H2O] = 0.20 M Keq = [0.10]2[0.20] [0.20] Keq = 0.010 3. If 0.600 mole of SO3 and 0.0200 mole of SO2 are found in a 2.00 L container at equilibrium at 25 oC. Calculate the [O2]. 2SO2(g) + O2(g) ⇌ 2SO3(g) Keq = 798 Keq [SO3] = 0.600 mole/2.00 L = 0.300 M [SO2] = 0.0200 mole/2.00 L = 0.0100 M = [SO 3]2 [SO2]2[O2] 798 1 = (0.3)2 = [O2] = (0.300)2 (0.0100)2[O2] 798(0.01)2[O2] (0.3)2 798(0.01)2 = 1.14 M Size of Keq & Effect of Temperature on Keq Big Keq products Keq = reactants Keq = 10 Little Keq products Keq = reactants Keq = 0.1 Note that the keq cannot be a negative number! Keq = 1 products Keq = reactants Effect of Temp on Keq Keq Only a temperature change can affect the value of Keq. Changes in concentrations, pressure or surface area have NO effect on Keq. – These changes correspond to increase in number of reacting molecules per liter. – Increased once and then equilibrium is re-established. – So ratios of products to reactants do not change. PBr3(g) + Br2(g) ⇋ PBr5(g) @ 100 oC + Keq = 0.17 energy @ 200 oC Keq = 0.091 How can you tell if Keq gets bigger or smaller? Temperature increased Shifted left, Keq decreased Keq= [products] ------------[reactants] More questions… If the value of Keq increases when the temperature decreases , is the reaction exothermic or endothermic? More questions… What will happen to the value of Keq in the following reaction if we added more [B]? A + B ⇌ C + 100 KJ. Hebden Practice Page 60: Exercises 31-35 Page 62: Exercises 36-45