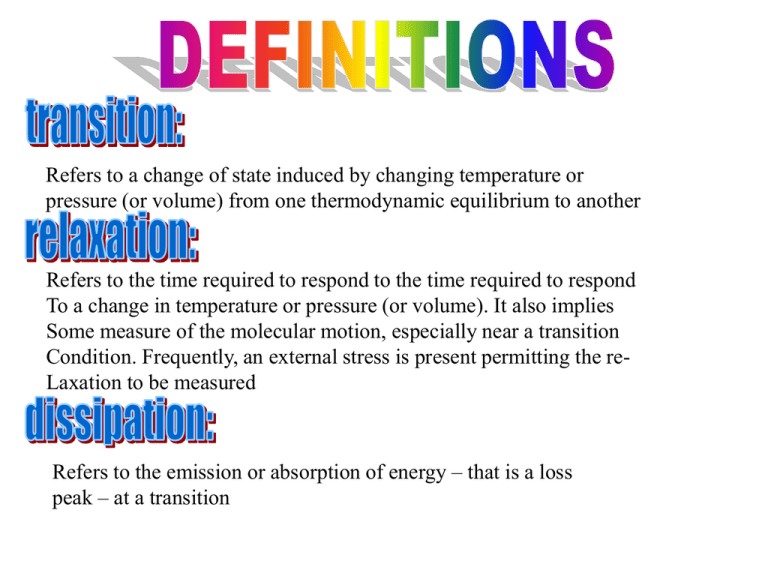
Refers to a change of state induced by changing temperature or
pressure (or volume) from one thermodynamic equilibrium to another
Refers to the time required to respond to the time required to respond
To a change in temperature or pressure (or volume). It also implies
Some measure of the molecular motion, especially near a transition
Condition. Frequently, an external stress is present permitting the reLaxation to be measured
Refers to the emission or absorption of energy – that is a loss
peak – at a transition
heat flux
or
cp
poly(dimethyl siloxane) (PDMS)
•glass transition
•enthalpy relaxation
•cold crystallization
•melting
H
H
dH
dT
T p , ni
p
dH p , n
i
n
H
dp
dni
n
i
1
T , ni
i p , T , n j i
H
dT
T p , ni
cp
ni ni 0 i dni i d
dH T , p i H
n
i 1
ni p ,T , n ji
i
H
T , p
d
n
i 1
i
i
differential enthalpyof reaction
c~p specificheatcapacity
c p molarheatcapacity
ni amountof subst ancecomponenti
i st oichiometriccoefficient of thecomponenti in a reaction
extendof reaction
i chemicalpot ent ialof thecomponenti
H
c~p
H
T
p
c~ dT dH
c~p dT
p
T
H
Q
c~p
Q
t p , n t p , n t p , n
i
i
i
T
T(t)
slope:
T
m *
t p
and from above :
1
Q
T
~
m * ~ Q c p
m*
t p c p
Cp can be calculated from the heat flux Q
Q is determined by the exactness of the signal
t
M*is determined by the exactness of the program rates
Consequently, a correction is needed that is independent of
the parameters of the instrument:
c~p Q sample Q sample msample
c~ Q sapphire Q sapphire msample
p
M. J. O`Neill, Anal. Chem. 38 (1966) 1331
Q
H
c~p T Ttrans H trans
t p
T p
Tg, Tm, Tcr, TS-N, TSA-SC,…TN-I…
Classification of the thermodynamic transitions
according to Paul Ehrenfest*):
Discontinuity the derivative (1st, 2nd,…) of the Gibbs function G
G H TS dG dH TdS SdT
G
G
dG
dp
dT
T p , n
p T , n
i
S
i
n
i 1
G
dni
ni p , n
V
dG T , S dH
*)Ehrenfest
P Proc Kon Akad Wetensch Amsterdam (1933) 36, 153
j i
i
First Order Transitions
In a real experiment heat transfer requires time
and a temperature gradient
and this is what we actually see:
a (endothermal) melting peak
Hoffman & Weeks Plot*
Tm0 Tm T 0mTc
thermodynamic equilibrium
melting temperature
stability parameter
isothermal crystallization temperature
experimental melting temperature
Tm
Tm0
Tm Tm0
Tm Tc
1
slope
Tm 1 Tm0 Tc
intercept
0 Tm Tm0 most stable
1 Tm Tc unstable
0.5 is the most common case
Tc
J. D. Hoffman, J. J. Weeks, J. Res. Bureau Standards 66A (1962) 13 *(solution crystl.)
Volume
Enthalpy
Storage modulus
At the glass transition
Expansivity
Heat capacity
Loss modulus
Vspec
Hspec
high cooling rate CR1
low cooling rate CR2
Tg2
Tg1
T
(dV/dT)~ a
(dH/dT)p= cp
Ti2 Tg2
Te2
Tg1
Tcr
Calculation of Tg according to
M. J. Richardson, N. G. Savill, Brit. Polym. J. 11 (1979) 123
Tg determined independent of the heating rate
integration
time












