Le Chatlier`s Principle
advertisement

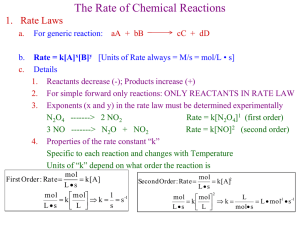
Le Chatlier’s Principle Subliminal message! Chemistry is great! Le Chatlier’s Principle When an equilibrium is disrupted, the reaction will shift to restore equilibrium. Subliminal message! Chemistry is great! Le Chatlier’s Principle The equilibrium can be affected by: - adding substances – the reaction will shift away from the change. - removing substances – the reaction will shift toward the change. Subliminal message! Chemistry is great! Le Chatlier’s Principle - temperature – acts like a reactant/product - container size – smaller container will shift toward small number of molecules - Adding inert gasses – no effect Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol 1) If pressure of A is increased Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol 1) If pressure of A is increased Reaction will shift right Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol 2) The temperature of the reaction is increased Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol The temperature of the reaction is increased Reaction will shift left 2) Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol A catalyst is added to the system 3) Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol A catalyst is added to the system No change in the equilibrium 3) Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol More compound B is steadily added to the reaction chamber 4) Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol 4) More compound B is steadily added to the reaction chamber The reaction will shift right Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol 5) An inhibitor is added Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol 5) An inhibitor is added No shift in equilibrium Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol Argon gas is added to the reaction chamber 6) Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol 6) Argon gas is added to the reaction chamber No shift in equilibrium Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol 7) The container is made smaller Subliminal message! Wednesdays and fridays at lunch in room 122 Le Chatlier’s Principle For example: Consider the reaction A+BC H = -453 kJ/mol 7) The container is made smaller the reaction will shift right