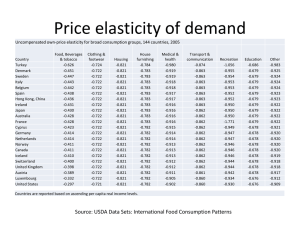
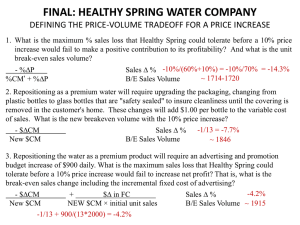
Resilin
advertisement

Image: http://www.murg.esm.vt.edu/research/images/L_murg-research-MPR-8.jpg The protein structure of resilin. Close examination reveals a lack of alpha helices and beta sheets that might be found in other elastomeric proteins. Introduction to Resilin 0 Elastomeric protein found in the elastic tendons and wing-hinges of many insects. 0 First discovered by Torkel Weis-Fogh in locust winghinge, paper published 1960.1 0 Within a year, resilin was discovered in the salivary pump of assassin bugs2 and in the feeding pump of Rhodnius prolixus3. It has been found in the wingfolding mechanism of Dermaptera earwigs recently.4 0 Seems to be nature’s protein version of a spring. This picture shows the location of the fluorescent resilin pad in the legs of the flea. The fluorescence is due to the dityrosine residues in the resilin protein. Image: http://www.voyle.net/Images%202005/Oct%202005/17 -10-2005-001.jpg Fleas are known to be able to jump over 150 times the length of their body, and resilin plays a key role in their jumping, as it is compressed and then releases the energy extremely efficiently to allow the flea to jump. The energy stored up from the leg muscles’ compression is released from resilin within 1 millisecond. Commercial Importance of Resilin 0 Most efficient elastomeric protein known to man currently, with a 97% efficiency in conversion of energy. 0 Natural resilin must last for the lifetime of insect, hence it must be able to be deformed and reformed millions of times without severe degradation. 0 Hence a possible substitute for rubber that is even superior to rubber and easier to produce. 0 Unmatched mechanical abilities: it demonstrated perfect elasticity even after being strained for twice its original length for two weeks, and had no tearing nor fatigue when stressed within its natural limits.1 Resilin’s Properties 0 Unaffected by deep freezing nor heating until 125°C, which is unusual for a protein. 0 Unaffected by alcohols and fixatives such as formalin or Bouin’s Solution (used for embryonic studies). 0 Energy loss at 200Hz under 5%5, compared to the previous best elastic material polybutadiene, which had an efficiency of 80%. 0 Unfortunately, resilin is degraded by proteolytic enzymes, hence artificial resilin will have to address this issue. Structure of Resilin 0 620 amino acids long, including a signal peptide of 17 residues at the N-terminus, suggesting that the protein is secreted into the extracellular space. 0 It has a relative molecular mass of 56771 daltons and a calculated isoelectric point of 5.0. 0 Composed of 3 domains: a central 62-residue domain, 324 residue long N-terminal region and a 217 residue long C-terminal. 0 Believed to exhibit elasticity because of immense cross-links composed of dityrosine and trityrosine, which also give it fluorescent properties.6 Autofluoresence under UV light from resilin present in male genitalia of a giant water bug, Belostoma lutarium. Image: http://jeb.biologists.org/content/210/22/3879/F3.large.jpg Molecular Basis for Resilin Elasticity 0 Resilin has a repeated 15-residue motif (with 18 slight variants) in the N-terminal region that is rich in glycine with 2 prolines and a tyrosine, GGRPSDSYGAPGGGN. 0 The C-terminal region possesses a 13-residue motif (with 11 slight variants) of glycine, tyrosine but only 1 proline, GYSGGRPGGQDLG. 0 These tyrosines are then used to form dityrosines and trityrosines which cross-link between peptide chains. Molecular Basis for Resilin Elasticity 0 Weis-Fogh was able to determine that elasticity of resilin was affected by the hydration and the pH of the protein.1 0 It has been suggested that another component of the elasticity comes from the hydration of the peptide chains. 0 Peptide groups will more easily hydrogen-bond to water than each other, causing a slightly irregular chain-folding that prevents cooperativity in inter-chain bonds, allowing limited links in the form of dityrosine and trityrosine links. 0 This creates a protein that has cross-links in 3D, rendering it highly compressible and efficient in energy transmission. Artificial Resilin 0 In a paper published in 2005, a group of Australian scientists led by Dr Chris Elvin of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) successfully synthesised resilin through genetic engineering. 0 The synthetic resilin was produced through isolation of the resilin gene from D. melanogaster and inserted into E. coli, which produced the precursor pro-resilin. 0 Pro-resilin was then mixed with ruthenium catalyst under a light which created tyrosine cross-links. Within 20 seconds, the texture changed into a rubbery solid with properties identical to natural resilin.7 Image: http://jeb.biologists.org/content/210/22/3879/F3.large.jpg Bio-synthesised resilin moulded into a flexible rod by drawing pro-resilin into a glass tube, followed by photochemical cross-linking of the precursor. Left, the rod illuminated by white light. Right, the same rod illuminated by U.V. light at 315 nm showing its fluorescence at 409 nm. Lucky Guess? 0 By studying other elastomeric proteins such as elastin, the CSIRO group reasoned that resilin was likely to have highly-repetitive sequences. 0 As such, the genes that coded for resilin was also likely to have highly-repetitive sequences. 0 Given that the gene that produced resilin was already identified, the researchers picked out a small section of DNA that contained large amounts of repeating elements in the hope that it would produce resilin. Insights into Resilin from Artificial Resilin 0 Closer study of the synthetic resilin eventually provided much information on the nature of resilin. 0 Secondary structure studies led by CSIRO scientists examined a 16-unit consensus sequence repeat, known as AN16.8 0 It was discovered to be structurally similar to many denatured proteins and intrinsically unstructured. Alphahelical and beta-sheets were not observed in the NMR spectroscopy. 0 Evidence was discovered through the NMR spectra that the Tyr-Gly-Ala-Pro sequence showed lower dependence on hydration, implying that the residues were involved in hydrogen-bond formation. Insights into Resilin from Artificial Resilin 0 The group concluded that the most likely model for resilin’s elasticity was a mix of random-network elastomers and sliding beta-turns. 0 This was further confirmed by a subsequent study which synthesised novel recombinant proteins with repetitive domains based off resilin genes in D. melanogaster and A. gambiae.9 0 The recombinant proteins produced were extremely similar to natural resilin in terms of modulus, elasticity, resilience and dityrosine content. Conclusion 0 While much headway has been made to understand the unique structural properties that give resilin such an unparalleled position in terms of elasticity and resilience, much work needs to be done to synthesise artificial resilin usable. 0 Potential applications include in spinal disc implants, heart and blood valve substitutes and high efficiency industrial rubbers. They could also be deployed in nanosprings and microactuators. 0 The major hurdle to overcome however, is its denaturation by proteases, as well as the effect of pH and hydration on its mechanical properties. References (I) 1 Weis-Fogh, T. (1960). A rubber-like protein in insect cuticle. J. Exp. Biol. 37,889 -907. 2 Edwards, J. S. (1960). Predation and digestion in assassin bugs (Heteroptera, Reduviidae). PhD thesis, University of Cambridge, UK. 3 Bennet-Clark, H. C. (1963). Negative pressures produced in the pharyngeal pump of the bloodsucking bug, Rhodnius prolixus. J. Exp. Biol. 40,223 -229. 4 Haas, F., Gorb, S. and Wootton, R. J. (2000). Elastic joints in dermapteran hind wings: materials and wing folding. Arthropod Struct. Develop. 29,137 -146. 5 Jensen, M. and Weis-Fogh, T. (1962). Biology and physics of locust flight. V. Strength and elasticity of locust cuticle. Phil. Trans. Roy. Soc. Lond. B 245,137 -169. References (II) O. (1964). The cross links in resilin identified as dityrosine and trityrosine. Biochim. Biophys. Acta 93,213 -215. 0 7 Elvin, C. M. et al (2005) Synthesis and properties of crosslinked recombinant pro-resilin. Nature, Oct 13 2005. Vol 437, No. 7061, pp. 999-1002 0 8 Nairn, K. M. et al (2008) A synthetic resilin is largely unstructured. Biophys J. 2008 Oct ;95(7):3358-65. Epub 2008 Jun 27. 0 9 Lyons, R. E. et al (2009) Comparisons of recombinant resilin-like proteins: repetitive domains are sufficient to confer resilin-like properties. Biomacromolecules. 2009 Nov 9;10(11):3009-14. 0 6 Andersen, S.