Enzyme Regulation: Allosteric Control & Modification
advertisement
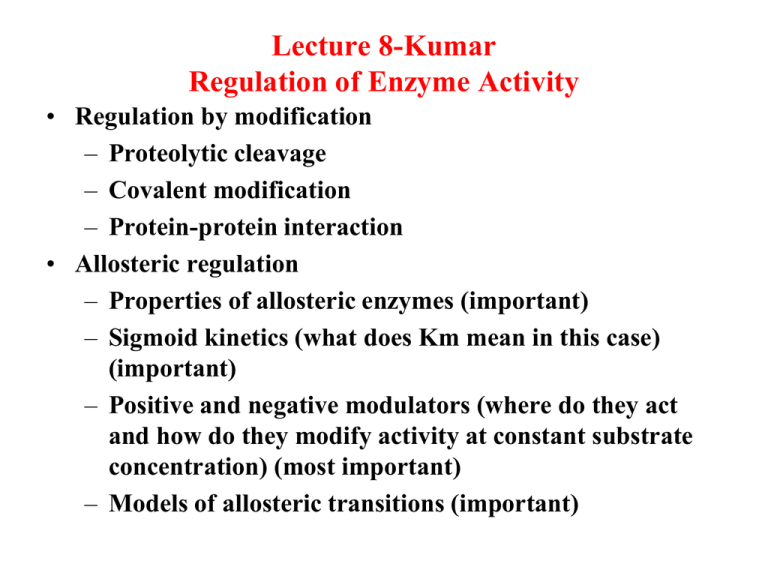
Lecture 8-Kumar Regulation of Enzyme Activity • Regulation by modification – Proteolytic cleavage – Covalent modification – Protein-protein interaction • Allosteric regulation – Properties of allosteric enzymes (important) – Sigmoid kinetics (what does Km mean in this case) (important) – Positive and negative modulators (where do they act and how do they modify activity at constant substrate concentration) (most important) – Models of allosteric transitions (important) Regulation of Enzyme Activity Normal metabolic control may be exerted in a variety of ways. Examples are: 1. Proteolytic Cleavage of inactive Proenzymes to active enzymes Pepsinogen pepsin + small peptide in gastrointestinal tract for protein digestion 2. Coagulation cascade—a series of proenzymes are converted to active enzymes. The last step is Fibrinogen Fibrin Coagulation Cascade Covalent Modification as Control Chemical modification can either increase or decrease activity. Some examples are: Glycogen Synthetase Phosphatase Kinase Phosphorylated Glycogen synthetase Glycogen phosphorylase Covalent Phosphorylated Glycogen phosphorylase Protein-Protein Interaction Example is activation of Protein Kinase A R2C2 + 4 cAMP R2C2(4cAMP) Inactive 2R(cAMP)2 + 2C (active) The catalytic unit (C) is able to phosphorylate and modulate the activity of other enzymes Allosteric Regulation Properties of Allosteric enzymes 1. 2. 3. 4. 5. 6. 7. Catalyze essentially irreversible reactions; are rate limiting Generally contain more than one polypeptide chain Do not follow Michaelis-Menten Kinetics Are regulated by allosteric activators or inhibitors Can be up-regulated by allosteric activators at constant [S] Can be down regulated by allosteric inhibitors at constant [S] Activators and Inhibitors need not have any structural resemblance to substrate structure Sigmoid kinetics for allosteric enzymes Effects of allosteric activators and allosteric inhibitors on enzyme activity Effect of allosteric activators and inhibitors on rate at cellular concentration of the substrate Models of Allosteric Modulation Symmetry model Sigmoidal Curve Effect vo Sigmoidal curve Noncooperative (Hyperbolic) ATP Positive effector (ATP) brings sigmoidal curve back to hyperbolic CTP Cooperative (Sigmoidal) Negative effector (CTP) keeps vo Exaggeration of sigmoidal curve yields a drastic zigzag line that shows the On/Off point clearly Consequently, Allosteric enzyme can sense the concentration of the environment and adjust its activity Off [Substrate] On Learning objectives for lecture 8 • Learn various methodologies that enzymes employ to control metabolism • Know the properties of an allosteric enzyme. • Understand the significance of sigmoid kinetics. Can one determine Km and Vmax for these enzymes from the sigmoid plot • Understand how the activity of an allosteric enzyme is regulated by allosteric activators and inhibitors • Understand the mechanism of allosterism and negative feedback inhibition