Some Common Types of Enzymes
advertisement
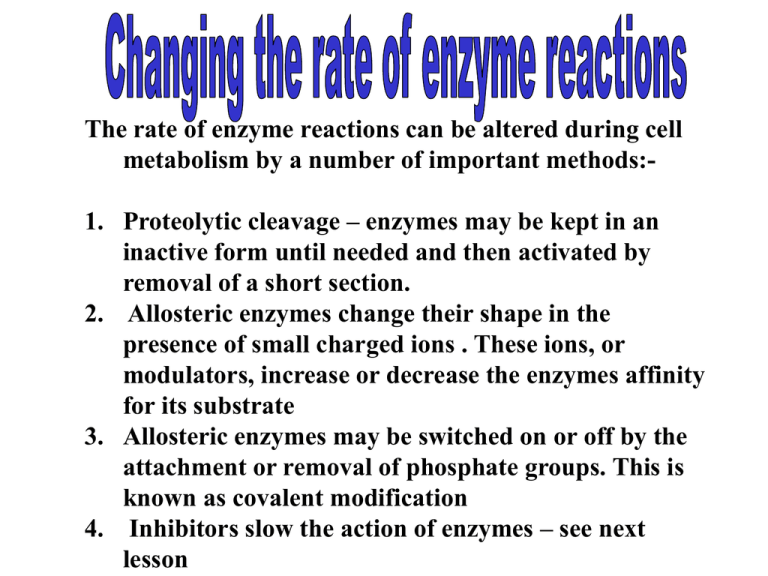
The rate of enzyme reactions can be altered during cell metabolism by a number of important methods:1. Proteolytic cleavage – enzymes may be kept in an inactive form until needed and then activated by removal of a short section. 2. Allosteric enzymes change their shape in the presence of small charged ions . These ions, or modulators, increase or decrease the enzymes affinity for its substrate 3. Allosteric enzymes may be switched on or off by the attachment or removal of phosphate groups. This is known as covalent modification 4. Inhibitors slow the action of enzymes – see next lesson Inactive enzymes can be activated by a process called Proteolytic cleavage. Example The pancreas secretes the inactive enzyme typsinogen into the duodenum. This is so that protein digestion does not start in the pancreas itself. An enzyme called enterokinase is secreted from the lining of the duodenum which processes the trypsinogen into its active form trypsin by cutting off part of the protein chain. The trypsin is able to digest the protein in food but also triggers the activation of more trypsinogen. Allosteric Enzymes and Modulators Small ions called modulators may bind to an allosteric enzyme, changing its shape in the process and also its affinity for its substrate. The modulator, also called the regulator or effector, can have a positive or negative effect on allosteric enzyme activity. · Positive modulators (activators) bind with an allosteric site on the enzyme and stabilise the ACTIVE conformation therefore speeding up the reaction rate Negative modulators (inhibitors) bind to an allosteric site that stabilises the INACTIVE conformation of the molecule, therefore slowing the reaction rate Examples of activators are: Ca2+ needed by thrombokinase. This converts globular prothrombin protein to fibrous thrombin protein which helps blood to clot. Cl- needed by salivary amylase. This hydrolyses starch to maltose during digestion. Summary of features associated with allosteric enzyme activity • Modulator molecules bind to a site on the enzyme which is remote from the active site • When a positive modulator binds, the enzyme shape is altered and the active site forms • When a negative modulator binds, the enzyme shape is altered and the active site no longer forms Another way of regulating enzyme activity is by covalent modification. This is achieved by covalent binding of a variety of chemical groups to the enzyme which changes the shape of the enzyme. Examples of covalent modification Addition or removal of a phosphate group with its 2 negative charges Kinase adds phosphate group to molecule while phosphatase removes it. It is reversible. Some enzymes are activated by phosphorylation others are inactivated. Example:- Muscle tissue needs glucose (in the form of glucose-1-phosphate) for energy. Glycogen is broken down by glycogen phosphorylase. The enzyme first needs to be activated by adding phosphate to a serine group using a kinase enzyme. When muscular activity slows down, a phosphatase acts on the phosphorylase to remove the phosphate group and inactivate the enzyme. Covalent Modification (e.g. with the enzyme glycogen phosphorylase) Inactive form of glycogen phosphate ACTIVE form of glycogen phosphate Covalent Modification of Enzymes (2) •Some enzymes are activated by phosphorylation others are inactivated.