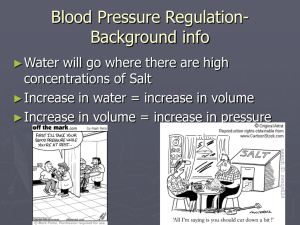
Diabetes Mellitus: Large volume of glucose-containing urine
Why is there glucose in the urine?
Why is urine volume increased?
Consider the following case:
Blood volume decreases, so we retain more water (i.e., what we
discussed during the previous class)
But that would cause dilution of the body fluids …
Tubular reabsorption of Na
Proximal Tubule
Distal Tubule
65%
3-5%
Filter ~ 1 lb of
Na daily
Loop of Henle
25-30%
Collecting Tubule
1-3%
Glomerulus
Proximal Tubule Sodium Reabsorption
Proximal Tubule
Epithelial Cell
Tubular Lumen
Capillary Lumen
(blood)
(urine)
Na+
2K+
ATP
H+
H+
3 Na+
Na+
glucose
amino acids
PO42-
Na+
H2O
3 HCO3-
OHCO2
Loop of Henle Sodium Reabsorption – thick ascending limb
Ascending Thick Limb of the
Loop of Henle Epithelial Cell
Tubular Lumen
Capillary Lumen
(blood)
(urine)
Na+
2K+
ATP
3 Na+
2 ClK+
K+ recycling
K+
+
Na+ Ca+2 Mg+2
Cl-
ROMK
channel
Paracellular Pathway
-
Point: if there are abnormalities in
the various transport processes
involved in Na+ reabsorption, there
are serious consequences in terms
of volume and osmotic regulation.
Ascending limb of
the loop of Henle
Bartter’s syndrome: hypotension
with salt wasting and hypokalemia as
a result of loss of Na reabsorption in
the thick ascending limb
Flatman (2008) Curr Opin Nephrol Hypertens 17: 186-192
Distal Tubule Sodium Reabsorption
Distal Tubule Epithelial Cell
Tubular Lumen
(urine)
Capillary Lumen
(blood)
Na+
2K+
ATP
Cl-
3 Na+
K+
Gitelman’s disease: a mutation in
the Na, Cl cotransporter; results in
sodium wasting and hypotension
Cl-
Collecting Tubule
Collecting Tubule Epithelial Cell
Principal Cell
Tubular Lumen
(urine)
Capillary Lumen
(blood)
2K+
ATP
Na+
3 Na+
K+
-
K+
+
Regulation of Tubular
Transport
•
•
•
+
Na
Hormones regulate the extracellular fluid volume by altering renal Na+
excretion
Hormones that enhance tubular Na+ reabsorption
– angiotensin II
– arginine vasopressin
– Aldosterone
Hormones that inhibit tubular Na+ reabsorption
– atrial natriuretic peptide
– natriuretic factors
• Ouabain
• Ouabain analogs
Actions of Aldosterone
(the simple version)
Silverthorn Figure 19-12
The key point of the next few slides is that the actions of aldosterone and the
regulation of Na+ transport are quite complex, and abnormalities in these regulatory
processes can result in diseases.
Model for the early transcriptional action of aldosterone on ENaC function.
The ubiquitin ligase Nedd4-2 that tonically inhibits ENaC surface expression is
highlighted in red, and red dashed arrows indicate pathways downregulating ENaC
that are antagonized by aldosterone. Blue boxes represent the regulatory proteins
implicated in the early aldosterone action that are rapidly induced via activated MR.
The blue lines terminated by a dash indicate at what level these regulatory proteins
interfere with ENaC inhibition.
Verrey et al. Kidney International (2008) 73: 691-696
Figure 1. Regulation of ENaC activity in the distal nephron Left-hand panel: Segments of the distal nephron
including the distal convoluted tubule (DCT; dark grey), connecting tubule (CNT; black), cortical collecting duct
(CCD; light grey) and medullary collecting duct (MCD; white) are shown. The juxtamedullary nephrons have a long
connecting tubule. Right-hand panel: Schematic of the principal cell of the connecting tubule or cortical collecting
duct. Aldosterone (Aldo) binds mineralocorticoid receptor (MR) in the nucleus to stimulate expression of several
genes: some important examples are shown. Aldosterone may also have non-genomic effects in the cortical
collecting duct/connecting tubule (not shown). Nedd4 and Nedd4-2 promote endocytosis and ubiquitination of
epithelial sodium channel (ENaC). Serum- and glucocorticoid-inducible kinase 1 (SGK1) regulates ENaC via
inhibition of Nedd4-2 and possibly by a direct mechanism. Furin and channel-activating protease (CAP) proteins
activate ENaC by proteolysis. Ub(n) is a polyubiquitin moiety and ER is the endoplasmic reticulum.
From: Thomas: Curr Opin Nephrol Hypertens, Volume 13(5).September 2004.541-548
Liddle’s mutations disrupt the interaction
between Nedd4 and ENaC
ENaC
Ubiquitination
Internalization
and Degradation
X
X
Ubiquitin
PY
X
N
C
N
PY
C
Nedd4
C2
WW Domains
Ubiquitin
Ligase
Ubiquitin
Ligase
PY
N
Mutations or Truncations
β or γ subunit
C
Liddle’s syndrome is an
autosomal dominant form of
salt sensitive hypertension
Expression of major sodium and potassium transport proteins in the distal nephron,
including WNK kinases and associated regulatory proteins.
Hoorn E J et al. JASN 2011;22:605-614
So, Aldosterone is important, but what controls aldosterone secretion?
AngII is the major stimulus
of aldosterone secretion
Silverthorn Figure 19-13
renin secreting cells of the
juxtaglomerular apparatus
Factors controlling renin secretion
Schematic depiction of the renin–angiotensin system components 2013
Only in tissue
Decarboxylation of asp
to ala in position 1
Point here: there is a lot more going on with the renin-angiotensin system
than just the classical stuff represented by the blue line in the figure.
Carey R. Hypertension 2013;62:818-822
Atrial Natriuretic Hormone
Germann figure 20.18
Silverthorn 19-15
Sympathetic influences
on renal function
G.F. DiBona
Fig. 1. Effects of increased renal sympathetic nerve activity
(RSNA) on the 3 renal neuroeffectors: the juxtaglomerular
granular cells (JGCC) with increased renin secretion rate (RSR)
via stimulation of 1-adrenoceptors (AR), the renal tubular
epithelial cells (T) with increased renal tubular sodium
reabsorption and decreased urinary sodium excretion (UNaV) via
stimulation of 1B-AR, and the renal vasculature (V) with
decreased renal blood flow (RBF) via stimulation of 1A-AR.
A quick word about ‘salt appetite’
At least in some species, Na+ deficit elicits the
motivation for salt seeking and increased salt intake
Issue of salt sensitivity of blood pressure, and dietary recommendations
Percentage change in mean arterial pressure in normotensive subjects receiving incremental
increases in sodium.
Weinberger M H Hypertension 1996;27:481-490
(based on data from Luft et al., 1979)
Copyright © American Heart Association
Percentage change in mean arterial pressure in normotensive subjects receiving incremental
increases in sodium.
Current
recommended
Current
US
average
Typical range for assessing salt sensitivity of BP
Weinberger M H Hypertension 1996;27:481-490
(based on data from Luft et al., 1979)
Copyright © American Heart Association
Low salt: 20 mmol/day (460 mg)
High salt: 240 mmol/day (5520 mg)
Flow chart of responses to
severe dehydration:
Silverthorn Figure 19-17
Now switching to K+
Figure 29-1 Normal potassium intake, distribution of potassium in the body fluids,
and potassium output from the body.
Figure 29-2 Renal tubular sites of potassium reabsorption and secretion.
Potassium is reabsorbed in the proximal tubule and in the ascending loop of
Henle, so that only about 8 per cent of the filtered load is delivered to the distal
tubule. Secretion of potassium into the late distal tubules and collecting ducts
adds to the amount delivered, so that the daily excretion is about 12 per cent of
the potassium filtered at the glomerular capillaries. The percentages indicate
how much of the filtered load is reabsorbed or secreted into the different tubular
segments.
ROMK
Figure 29-3 Mechanisms of potassium secretion and sodium reabsorption by the
principal cells of the late distal and collecting tubules.
Figure 29-4 Effect of plasma aldosterone concentration (red line) and
extracellular potassium ion concentration (black line) on the rate of urinary
potassium excretion. These factors stimulate potassium secretion by the
principal cells of the cortical collecting tubules. (Drawn from data in Young DB,
Paulsen AW: Interrelated effects of aldosterone and plasma potassium on
potassium excretion. Am J Physiol 244:F28, 1983.)
Figure 29-5 Effect of extracellular fluid potassium ion concentration on plasma
aldosterone concentration. Note that small changes in potassium concentration
cause large changes in aldosterone concentration.
Figure 29-8 Effect of large changes in potassium intake on extracellular fluid
potassium concentration under normal conditions (red line) and after the
aldosterone feedback had been blocked (blue line). Note that after blockade of the
aldosterone system, regulation of potassium concentration was greatly impaired.
(Courtesy Dr. David B. Young.)
Figure 29-9 Effect of high sodium intake on renal excretion of potassium. Note
that a high-sodium diet decreases plasma aldosterone, which tends to
decrease potassium secretion by the cortical collecting tubules. However, the
high-sodium diet simultaneously increases fluid delivery to the cortical
collecting duct, which tends to increase potassium secretion. The opposing
effects of a high-sodium diet counterbalance each other, so that there is little
change in potassium excretion.
Model of the “aldosterone paradox.” Two pathophysiological settings
are depicted: hypovolemia (left) and hyperkalemia (right).
Hoorn E J et al. JASN 2011;22:605-614
The key point to take away from this is that the overall effect on Na+ reabsorption
versus K+ secretion in the nephron is dependent upon whether the increase in
aldosterone occurs with or without an increase in AngII.
A word about Ca++ homeostasis
Regulation of parathyroid hormone secretion by Ca++
Point: PTH secretion is directly sensitive to changes in blood Ca++
Diuretic Drugs: (see table 31-1 in Guyton)
•Osmotic diuretics (e.g., mannitol)
•Loop diuretics (e.g., furosemide)
•Thiazide diuretics (e.g., hydrochlorothiazide)
•Aldosterone antagonists (e.g., spironolactone)
•Drugs that block Na channels in the collecting ducts (e.g., amiloride)
•Carbonic anhydrase inhibitors (e.g., acetazolamide)
(notice that ADH antagonists are not on the list. Why?)
Is coffee a diuretic?
(Caffeine intake ~ 300 mg/day)
TBW measured by dilution of D2O
In higher doses, caffeine is a diuretic; its action is mostly on the proximal tubule to
reduce Na+ reabosorpton
Journal of Pharmacology and Experimental Therapeutics 313: 403-409, 2005
Caffeine dose: 45 mg/kg oral
~5-7 cups of coffee per day
A1 knockout mice
And to put this back into physiology,
the adenosine A1 receptors are
Macula densa
required for the signaling of
tubuloglomerular feedback!
J. Schnermann and J.P Briggs
Effect of Renal Sympathetic Denervation on Glucose Metabolism in Patients
With Resistant Hypertension Clinical Perspective
Felix Mahfoud, Markus Schlaich, Ingrid Kindermann, Christian Ukena, Bodo Cremers,
Mathias C. Brandt, Uta C. Hoppe, Oliver Vonend, Lars C. Rump, Paul A. Sobotka, Henry
Krum, Murray Esler, and Michael Böhm
Mahfoud F et al. Circulation. 2011;123:1940-1946
Copyright © American Heart Association, Inc. All rights reserved.
Effects of increased sympathetic activity on peripheral circulation and organs.
Böhm M et al. Circulation Research. 2014;115:400-409
Copyright © American Heart Association, Inc. All rights reserved.
Effects on plasma constituents of shutting down the kidneys
(NPN = nonprotein nitrogen)
Renal Dialysis
Table 31-7. Comparison of Dialyzing Fluid with Normal
and Uremic Plasma
Dialyzing
Fluid
Uremic
Plasma
142
133
142
K+
5
1.0
7
Ca++
3
3.0
2
Mg++
1.5
1.5
1.5
Cl-
107
105
107
HCO3-
24
35.7
14
Lactate-
1.2
1.2
1.2
HPO4=
3
0
9
Urate-
0.3
0
2
Sulfate=
0.5
0
3
Glucose
100
125
100
Urea
26
0
200
Creatinine
1
0
6
Constituent
Normal
Plasma
Electrolytes (mEq/L)
Na+
Nonelectrolytes