Vascular Complications - The 6th Arab Diabetes Forum In
advertisement

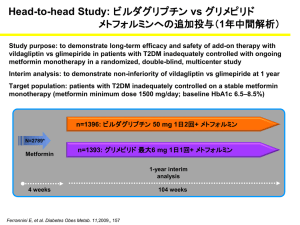
Management of Diabetes Mellitus during Ramadan By Professor Megahid M, Abuelmagd Diabetes and Endocrinology Unit Mansoura University DIABETES & RAMADAN Pathophysiology of Fasting in normal individuals Peripheral tissues (muscle) Gluconeogenesis Counteregulat ory hormones Increased glucose uptake Glucose FFA, Ketones Liver Glycogen stores depleted Pancreas insulin secretion decreased Adipose Pathophysiology of Fasting in Diabetes Hyperglycaemia &Ketoacidosis Gluconeogenesis & Peripheral tissues (muscle) ketogenesis Counteregulat ory hormones Increased glucose uptake Glucagon Glucose FFA, Ketones Liver Glycogen stores depleted Excessive breakdown Pancreas insulin secretion decreased or absent Adipose THE NEED FOR BALANCE HYPERGLYCEMIA Diabetes-associated risks Adjusted incidence per 1000 person years (%) 70 Microvascular endpoint 60 50 Myocardial infarction 40 30 20 10 0 5 6 7 8 9 10 11 Mean HbA1c (%) UKPDS 35. BMJ 2000; 321: 405-12 10 Pathophysiology Hyperglycemia Oxidative Stress Atheroma Formation Endothelial Dysfunction Thrombus Formation Vascular Complications 11 Vascular Complications 3 years incidence (%) 50 vascular complications among diabetics 45 40 CONTROL n= 35620 35 DM n=8905 30 25 20 15 10 5 0 MI HTN Ramsey, pharmacoeconomics, 1999 FOOT ULCER END STAGE RENAL DISEASE STROCK DM RELATED EYE DISEASE 12 Cost of vascular complications Micro vascular complications X 1.7 Macro vascular complications X 3.0 Macro & Micro X 3.5 13 Causes of death in diabetes % of deaths in diabetes mainly due to vascular complications 45 40 35 30 25 20 15 10 5 0 IHD OTHER HD DM CANCER STROKE INFECTIONS OTHERS 14 Main risk associated with fasting in diabetic patients is: Hypoglycemia Hypoglycemia Decreased food intake is a well-known risk factor for the development of hypoglycemia. Results of the Diabetes Control and Complications Trial (DCCT) showed a threefold increase in the risk of severe hypoglycemia in patients who were in the intensively treated group and had an average HbA1c (A1C) value of 7.0% Hypoglycemia The incidence of severe hypoglycemia was probably underestimated in this study, since events requiring assistance from a third party without the need for hospitalization were not included. Sever hypoglycemia was more frequent in patients: • • in whom the dosage of oral hypoglycemic agents or insulin were changed. in those who reported a significant change in their lifestyle Hypoglycemia and clinical implications • The ultimate goal of the glycemic management of diabetes is a lifetime of euglycemia without hypoglycemia (1) • Hypoglycemia is recognized to be a major limitation in achieving good control (2) 1. American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28(5):1245-1249 2. Cryer PE. Diabetologia. 2002;45:937–948 Physiological defenses against falling plasma glucose concentrations Adapted from: Cryer PE. J Clin Invest. 2006;116:1470–1473 Hypoglycemia impairs defenses against recurrent hypoglycemia (Hypoglycemia-Associated Autonomic Failure) Antecedent hypoglycemia Sleep Antecedent exercise Reduced sympathoadrenal responses to hypoglycemia Reduced sympathetic neural responses Reduced epinephrine responses Defective glucose counter regulation Hypoglycemia unawareness Recurrent hypoglucemia Cryer PE. J Clin Invest. 2006;116:1470–1473 Mechanisms by which hypoglycemia may affect cardiovascular events Desouza CV, et al. Diabetes Care. 2010; 33:1389–394 Classification of hypoglycemia according to severity: European Committee for Medicinal Products for Human Use (CHMP) Episodes suggestive of hypoglycemia where blood glucose measurement were not available. Minor hypoglycemic episodes defined as either a symptomatic episode with blood glucose level below 3 mmol/L [54mg/dl] and no need for external assistance, or an asymptomatic blood glucose measurement below 3 mmol/L, Major hypoglycemic episodes defined as symptomatic episodes requiring external assistance due to severe impairment in consciousness or behaviour, with blood glucose level below 3 mmol/L and prompt recovery after glucose or glucagon administration, Guideline on clinical investigation of medicinal products in the treatment of diabetes mellitus . CPMP/EWP/1080/00 Rev. 1 . Committee for Medicinal Products for Human Use (CHMP) . 20 January 2010 . Risk Difference of Hypoglycemia with Different Glucose-lowering Agents for T2DM Drug 1 less harmful Drug 1 more harmful Pooled effect (95% CI) Studies (participated) Met vs Met + TZD 0.00 (-0.01 to 0.01) 3 (1557) SU vs repag 0.02 (-0.02 to 0.05) 5 (1495) Glyb vs other SU 0.03 (0.00 to 0.05) 6 (2238) SU vs Met 0.04 (0.0 to 0.09) 8 (2026) SU + TZD vs SU 0.08 (0.00 to 0.16) 3 (1028) SU vs TZD 0.09 (0.03 to 0.15) 5 (1921) SU + Met vs SU 0.11 (0.07 to 0.14) 8 (1948) SU + Met vs Met 0.14 (0.07 to 0.21) 9 (1987) 0 0.5 0.15 0.15 0.2 Weighted absolute risk difference CI=confidence interval; Glyb=glyburide; Met=metformin; repag=repaglinide; SU=sulfonylurea; TZD=thiazolidinediones. Bolen S, et al. Ann Intern Med. 2007;147:386–399 Relative Risk of Hypoglycemia with Different Glucoselowering Agents when added to Metformin HbA1c Goal Achieved Group vs. Placebo No. of Trials RR (95% CI) Hypoglycemia No. of Trials RR (95% CI) All drugs 10 2.56 (1.99 to 3.28)b 19 1.43 (0.89 to 2.30) Sulfonylureas 1 3.38 (2.02 to 5.83)a 3 2.63 (0.76 to 9.13)a Glinides 1 3.20 (1.47 to 7.58) 2 7.92 (1.45 to 43.21) Thiazolidinedione s 1 1.69 (1.24 to 2.33) 2 2.04 (0.50 to 8.23) AGIs 0 2 0.60 (0.08 to 4.55) DPP-4 inhibitors 6 2.44 (1.78 to 3.33)b 8 0.67 (0.30 to 1.50) GLP-1 analogs 1 3.96 (2.37 to 6.79) 2 0.94 (0.42 to 2.12) NA Adapted from: Phung, et al. JAMA. 2010;303(14):1410–1418 Abbrevations: AGIs, α-glucosidase inhibitors; CI, confidence interval; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; HbA1c, glycated hemoglobin A1c; NA, not applicable; RR, relative risk; WMD, weighted mean difference. a ≥ 75% b = 50%-75% UKPDS – Treating to Targets Elevates the Risk of Hypoglycemia and Incidence can be High with SUs Cumulative Incidence of Hypoglycemia in T2DM over 6 Years 76 80 Sulfonylurea (n=922) 70 Insulin (n=689) Patients (%) 60 50 45 40 30 20 11.2 10 3.3 0 Sulfonylurea Insulin Any hypoglycema Sulfonylurea Insulin Major hypoglycemia* HbA1c = 7.1% in all groups SUs=sulfonylureas; T2DM=type 2 diabetes melllitus; *Requiring medical assistance or hospital admission UK Prospective Diabetes Study Group. Diabetes.1995;44:1249–1258. Relative Risk (%) Risk of Hypoglycemia with Different Sulfonylureas Severe hypoglycemia* n/1000 person years = Gliclazide 0.85 Glipizide 8.70 *<50 mg/dL. Tayek J. Diabetes Obes Metab. 2008;10:1128–1130. Glimepiride 0.86 Tolbutamide 3.50 Chlorpropamide 16.00 Glyburide 16.00 Health and economical consequences of hypoglycemia Hospitalization costs1 CV complications2 Weight gain by defensive eating3 Dizzy turn unconsciousness2 Hypoglycemia Seizures2 Car accident4 Increased risk of dementia5 Death6 Coma2 Quality of Life7 1. Jönsson L, et al. Cost of Hypoglycemia in Patients with Type 2 Diabetes in Sweden. Value In Health. 2006;9:193–198 2. Barnett AH. CMRO. 2010;26:1333–1342 3. Foley J & Jordan. J. Vasc Health Risk Manag. 2010;6:541–548 4. Canadian Diabetes Association’s Clinical Practice Guidelines for Diabetes and Private and Commercial Driving. CanJ Diabetes. 2003;27(2):128 –140. 5. Whitmer RA, et al. JAMA. 2009;301:15655–1572 6. Zammitt NN, et al. Diabetes Care. 2005;28:2948–2961 7. McEwan P, et al. Diabetes Obes Metab. 2010;12:431–436 Hypoglycemia and Weight Gain are intertwined Foley J, et al. Vasc Health Risk Manag. 2010:6 541–548 Impact of changes in weight and rates of hypoglycaemia events on Quality-Adjusted Life Year (QALY) McEwan, et al. Diabetes Obes Metab. 2010;12:431–436 Vildagliptin improves Alpha and Beta Cell selectivity for both Hyper and Hypoglycemia Vildagliptin improves β-cell sensitivity to glucose Glucose Sensitivity (pmol/min/m2/mM) 75 70 65 60 55 50 45 −4 0 4 8 12 16 20 24 28 32 36 40 44 48 52 Time (weeks) Basal Secretory Tone Secretion at 7 mM glucose (pmol/min/m2) Glucose Sensitivity 260 240 220 200 180 −4 0 4 8 12 162024283236 40444852 Time (weeks) Vildagliptin 50 mg once daily Placebo Mari A, et al. J Clin Endocrinol Metab. 2008; 93: 103–109. Effects of vildagliptin treatment on the sensitivity of the α-cell to glucose During the hypoglycemic steps, glucagon levels increased from a significantly lower baseline to a slightly higher level with vildagliptin compared with placebo. 170 Meal 7.5 mM 5.0 mM 2.5 mM Dose Glucagon (mg/L) 150 130 110 90 −30 0 30 60 Ahrén B, et al. J Clin Endocrinol Metab. 2009;94(4):1236–1243. Vildagliptin 100 mg once daily is NOT an approved dose. 90 120 165 Time (min) 210 255 285 Vildagliptin 100 mg once daily Placebo Comparing with commonly used SUs In patients uncontrolled with metformin monotherapy vildagliptin is as effective as glimepiride over 1 year with low incidence of hypoglycaemia and no weight gain Duration: 52 weeks, add-on to metformin: vildagliptin vs glimepiride Mean HbA1c reduction a Incidence of hypoglycaemia Number of hypoglycaemic events Patients with 1 hypos (%) 7.5 n = 1389 1383 1389 1383 Number of severe hypoglycaemic events c 1389 1383 554 −0.4% 6.7 −0.5% 6.5 No. of events 6.9 16.2 No. of events NI: 97.5% CI (0.02, 0.16) Incidence (%) 7.1 0.0 -8 -4 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 1.7 39 Time (weeks) Change in body weight a Vildagliptin 50 mg twice daily + metformin Glimepiride up to 6 mg once daily + metformin (BL mean ~88.8kg) Adjusted mean change in body weight (kg) from BL Mean HbA1c (%) 7.3 b BL=baseline; CI=confidence interval NI=non-inferiority; aPer protocol population ; bSafety population. cGrade 2 or suspected grade 2 events. *P <0.001; adjusted mean change from BL to Week 52, between-treatment difference and P value were from an ANCOVA model containing terms for treatment, baseline and pooled centre. n= 1117 1071 Ferrannini E et al. Diab Obes Metab 2009; 11: 157–166. Vildagliptin was as effective as glimepiride when added to metformin at 2 years with no weight gain and low incidence of hypoglycemia Duration: 104 weeks, add-on to metformin: vildagliptin vs glimepiride Hypoglycaemia Number of hypo events N = 1553 1546 N= 15 No. of events 4 2 0 0 Vildagliptin 50 mg bid + met Glimepiride up to 6 mg qd +met Change from BL to EP (BL Mean ~89kg) Adjusted mean change in body weight (kg) Primary objective of non-inferiority was met: 97.5% CI= (-0.00, 0.17); upper limit 0.3% 16 14 12 10 8 6 4 2 0 13 0 Change in body weight 3 1 Adjusted mean change in HbA1c was comparable between vildagliptin and glimepiride treatment: −0.1% (0.0%) for both N = 1553 1546 12 10 8 6 59 Mean HbA1c Discontinuations due to hypos 1553 1546 16 14 No. of events N = 1553 1546 18.2 Incidence (%) Number of severe events a No. of events Patients with > 1 hypo (%) 2 n = 1539 Between-treatment Difference 1520 1.2 2.0 1.0 0.0 -1.0 -2.0 -0.3 -1.5 * 1) Per protocol population. 2) Safety population. 3) Intent-to-treat population. a) any episode requiring the assistance of another party *p <0.001. BL=baseline; EP = week 104 endpoint; Met= metformin; hypo = hypoglycemia; HbA1c= glycosylated hemoglobin. Matthews DR et al. Diab Obes Metab 2010; 12: 780–789. Very elderly patients pooled analysis Very elderly patients pooled analysis: Vildagliptin add on metfrmin shows NO hypoglycemic event Pooled analysis at 24 weeks; 50 mg bid HbA1c Reduction: At 24 weeks treatment n= 62 25 BL 8.3 8.5 * *<0.05 vs baseline (within group) Monotherapy studies pool Add on therapy studies pool n= 62 25 BL 74.9 82.8 Change in Body Weight (kg) from baseline Add on therapy studies pool Change in HbA1c (%) from baseline Monotherapy studies pool Body Weight: At 24 weeks treatment * Mono > 75 * Add on > 75 Hypoglycemic events Monotherapy studies pool Add on therapy studies pool Any events 0.0 0.0 Severe events 0.0 0.0 OR No hypoglycemic events, including severe events was reported in elderly patients with monotherapy and add-on therapy Schweizer A. et al, Diabetes, Obesity and Metabolism 13: 55–64, 2011. Even When Add-on to Insulin Fewer hypoglycemic events in vildagliptin add-on to insulin compared with insulin alone Add-on Treatment to Insulin Duration: 24 weeks Add-on to insulin: vilda vs PBO No. of Events No. of Severe Events 185 No. of Events 200 160 120 113 80 40 0 No. of Severe Events * 10 ** 8 6 6 4 2 0 0 Vilda 50 mg twice daily + insulin PBO + insulin PBO=placebo; vilda=vildagliptin; *P <0.001; **P <0.05 between groups. Fonseca V, et al. Diabetologia. 2007; 50: 1148–1155. The Question Now: Is Vildagliptin A Safe Drug To Be Used With Diabetic Patients During Rmadan VECTOR: Aim and objectives Vildagliptin Experience Compared To gliclazide Observed during Ramadan Main aim To determine the incidence of hypoglycaemic events in Muslim patients with T2D fasting during Ramadan, who are treated with dual therapy of metformin plus vildagliptin or metformin plus sulphonylurea (SU) Primary objectives The incidence of hypoglycaemic events defined as: Any reported symptoms by the patient and/or any blood glucose measurement of less than 3.9 mmol/L (also defined as mild or Grade 1 hypoglycaemia) The need for third party assistance (also defined as severe or Grade 2 hypoglycaemia); Secondary objectives The change in weight; The change in HbA1c levels; and The treatment adherence during Ramadan. M. Hassanein et al Current Medical Research & Opinion Vol. 27, No. 7, 2011, 1367–1374 Study Design Observational, Non-interventional Two-cohort study Conducted in the UK. HbA1c 8.5% up to 1 month prior to fasting -----Data collection Metformin 2000 + Vildagliptin 50 mg bid daily Metformin 2000 + 6weeks pre Ramadan Gliclazide 80 mg* per daily Ramadan n23 n 36 6 weeks post Ramadan *Different formulations were used for gliclazide therefore the following conversion factor was used: 80 mg standard formulation 30 mg modified release formulation. M. Hassanein et al Current Medical Research & Opinion Vol. 27, No. 7, 2011, 1367–1374 Vildagliptin: Significantly lowered HbA1c compared to SU Change in HbA1c (%) from baseline Duration: ≤16 week observational study Add-on to metformin: vildagliptin vs gliclazide BL= 0.1 7.6 7.2 + 0.1 0.0 p= 0.02* -0.2 Vildagliptin 50 mg twice daily (n=23) Sulfonylurea (n=36) -0.4 -0.4 Hypoglycaemic events Vildagliptin Sulfonylurea Any events 0 34 (in 15 pts) Severe events 0 1 *mean between-group difference 0.5% BL=baseline; HbA1c=haemoglobin A1c Hassanein M, et al. Curr Med Res Opin 2011; 27: 1367–1374 VECTOR Study: Results No. of Hypos P=0.0002 Hypoglycaemia • Vildagliptin arm no Hypo • SU arm - 34 Hypo including 1 severe HE M. Hassanein et al Current Medical Research & Opinion Vol. 27, No. 7, 2011, 1367–1374 VECTOR Study: Results Adherence Vildagliptin arm : 0.2 missed doses (p=0.0204) SU arm : 7.6 missed doses Weight Body weight remained unchanged in both groups M. Hassanein et al Current Medical Research & Opinion Vol. 27, No. 7, 2011, 1367–1374 Consistency of Data Devendra et al. Int J Clin Pract. 2009;63(10):1446– 1450 Devendra et al. Int J Clin Pract. 2009;63(10):1446–1450 Methods HbA1c was > 8.5% despite treatment with metformin 2 g daily before Ramadan All patients received education about how to identify and manage hypoglycemia during Ramadan. Vildagliptin 50 mg bid daily n26 N= 52 Metformin 2 g StudyDesign Design Study Gliclazide 160 mg bd n 26 2 weeks Ramadan 10 days after Ramadan Recording of hypoglycemia and weight gain Devendra et al. Int J Clin Pract. 2009;63(10):1446–1450 Vildagliptin therapy and hypoglycaemia in Muslim T2DM during Ramadan Glycated haemoglobin (%) Hypoglycemic events n= 26 26 n= 26 26 BL 8.98 8.95 BL 0.42 0.27 0.6 0.42 -0.5 -1 -1.5 -1.26 * -2 -2.5 -3 *P=0.8217; **P=0.0168 -1.23 LSM change in hypoglycemic events LSM change in HbA1c 0 0.4 0.2 0 -0.2 -0.24 ** -0.4 Vildagliptin Gliclazide Analysis of covariance models with treatment group, gender and ethnicity as factors. aAge, duration of diabetes, HbA1c, weight and prefasting value, bage, duration of diabetes, weight and prefasting value, or cage, duration of diabetes, HbA1c and prefasting value as covariates. SD, standard deviation; SEM, standard error mean. Devendra et al. Int J Clin Pract. 2009;63(10):1446–1450 Diabetes Care, Diabetologia. 19 April 2012 [Epub ahead of print]