Diabetic Ketoacidosis
advertisement

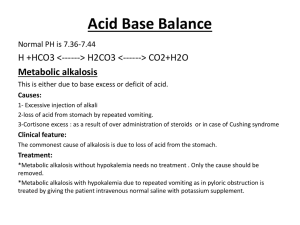
Ted A. Bonebrake, M.D. A 14 y/o female is brought to the emergency department by her mother after being found unresponsive at home. She had been ill the day before with nausea and vomiting, but was not running a fever. Her parents had kept her home from school that day. When her mother came home at lunchtime to check on her, she was very lethargic and not responding coherently. By the time she arrived at the hospital, she had to be brought in to the ED on a gurney. Initial evaluation showed O2 sat 100% on room air, pulse 126, respirations 30, BP 92/68, temperature 101.2 F. She appears pale, mucous membranes are dry and she only responds to painful stimuli. Exam shows diffuse abdominal tenderness with guarding. Differential diagnosis? What initial treatment would you suggest? What labs would you order? Any xrays or additional studies? CBC WBC 23,500 Hgb 14.2 g/dL Hct 45% Platelets 425,000 BMP Sodium 126 Potassium 5.2 Chloride 87 CO2 <5 BUN 32 Creatinine 1.5 Glucose 1,376 Arterial Blood Gases pH 7.19 Po2 100 mm Hg HCO3 7.5 mmo/L Pco2 20 mm Hg Sao2 98% (room air) Urine Specific gravity 1.015 Ketones 4+ Leukocytes few Glucose 4+ Nitrates 0 RBCs many Diabetic ketoacidosis (DKA) is an acute metabolic complication of diabetes characterized by hyperglycemia, hyperketonemia, and metabolic acidosis. DKA occurs mostly in type 1 diabetics. It causes nausea, vomiting, and abdominal pain and can progress to cerebral edema, coma, and death. DKA is diagnosed by detection of hyperketonemia and anion gap metabolic acidosis in the presence of hyperglycemia. Treatment involves volume expansion, insulin replacement, and prevention of hypokalemia. Diagnosis Epidemiology Pathophysiology Treatment Initial (emergency) treatment Ongoing management and monitoring Prognosis Symptoms and signs of DKA Nausea & vomiting Abdominal pain--particularly in children Lethargy and somnolence Kussmaul respirations Hypotension Tachycardia Fruity breath due to exhaled acetone Fever +/- if present may signify underlying infection In the absence of timely treatment, DKA progresses to coma and death. Arterial pH Serum ketones Calculation of anion gap Electrolytes, BUN and creatinine, glucose, ketones, and osmolarity should be measured Urine ketones Patients who appear significantly ill and those with positive ketones should have ABG measurement. DKA is diagnosed by an arterial pH < 7.30 with an anion gap > 12 and serum ketones in the presence of hyperglycemia. A presumptive diagnosis can be made when urine glucose and ketones are strongly positive. Calculation Anion Gap AG = ([Na+] + [K+]) − ([Cl−] + [HCO3−]) Or omit potassium AG = [Na+] − ([Cl-] + [HCO3−]) Alcoholic Ketoacidosis Appendicitis, Acute Hyperosmolar Coma Hypophosphatemia Hypothermia Lactic Acidosis Metabolic Acidosis Myocardial Infarction Pancreatitis, Acute Pneumonia, Immunocompromised Septic Shock Salicylate Toxicity Urinary Tract Infection DKA accounts for 50% of diabetes-related admissions in young persons and 1-2% of all primary diabetes-related admissions. DKA frequently is observed during the diagnosis of type 1 diabetes and often indicates this diagnosis. While the exact incidence is not known, it is estimated to be 1 out of 2000. DKA occurs primarily in patients with type 1 diabetes. The incidence is roughly 2 episodes per 100 patient years of diabetes. About 3% of patients with type 1 diabetes initially present with DKA. It can occur in patients with type 2 diabetes as well; this is less common, however. The incidence of DKA is higher in whites because of the higher incidence of type 1 diabetes in this racial group. The incidence of diabetic ketoacidosis (DKA) is slightly greater in females than in males for reasons that are unclear. Recurrent DKA frequently is seen in young women with type 1 diabetes and is caused mostly by the omission of insulin treatment. Among persons with type 1 diabetes, DKA is much more common in young children and adolescents than it is in adults. DKA tends to occur in individuals younger than 19 years, but it may occur in patients with diabetes at any age. Multiple factors (eg, ethnic minority, lack of health insurance, lower body mass index, preceding infection, delayed treatment) affect the risk of developing DKA among children and young adults Diabetic ketoacidosis (DKA) is a complex disordered metabolic state characterized by hyperglycemia, ketoacidosis, and ketonuria. DKA occurs as a consequence of absolute or relative insulin deficiency that is accompanied by an increase in counter-regulatory hormones (ie, glucagon, cortisol, growth hormone, epinephrine). The hormonal imbalance enhances hepatic gluconeogenesis, glycogenolysis, and lipolysis. Hepatic gluconeogenesis, glycogenolysis secondary to insulin deficiency, and counterregulatory hormone excess result in severe hyperglycemia Lipolysis increases serum free fatty acids. Hepatic metabolism of free fatty acids as an alternative energy source (ketogenesis) results in accumulation of acidic intermediate and end metabolites (ketones). Ketones include acetone, beta-hydroxybutyrate, and acetoacetate. Increased concentration of ketones initially leads to a state of ketonemia. Extracellular and intracellular body buffers can limit ketonemia in its early stages, as reflected by a normal arterial pH associated with a base deficit and a mild anion gap. When the accumulated ketones exceed the body's capacity to extract them, ketonuria results. If the situation is not treated promptly, this leads to clinical metabolic acidosis (ketoacidosis) Respiratory compensation for the acidosis results in rapid shallow breathing (Kussmaul respirations) Ketones induce nausea and vomiting that aggravate fluid and electrolyte loss already existing in DKA. Acetone produces the fruity breath odor that is characteristic of ketotic patients. Hyperglycemia, osmotic diuresis, serum hyperosmolarity, and metabolic acidosis result in severe electrolyte disturbances. The most characteristic disturbance is total body potassium loss Potassium loss is caused by a shift of potassium from the intracellular to the extracellular space in an exchange with hydrogen ions that accumulate extracellularly in acidosis. Potassium is lost in urine because of osmotic diuresis. Patients with initial hypokalemia are considered to have severe total body potassium depletion. High serum osmolarity drives water from intracellular to extracellular space causing dilutional hyponatremia. Sodium also is lost in the urine. In the absence of insulintissues such as muscle, fat, and liver do not take up glucose. Counterregulatory hormones, such as glucagon, growth hormone, and catecholamines, enhance triglyceride breakdown into free fatty acids and gluconeogenesis. This causes the elevation in serum glucose level in DKA. Beta-oxidation of these free fatty acids leads to increased formation of ketone bodies. Metabolism in DKA shifts from the normal fed state characterized by carbohydrate metabolism to a fasting state characterized by fat metabolism. This results in metabolic acidosis as the ketone bodies produced by beta-oxidation of free fatty acids deplete extracellular and cellular acid buffers. Osmotic diuresis depletes sodium, potassium, phosphates, and water, as well as ketones and glucose. Typical free water loss in DKA is approximately 6 liters or nearly 100 mL/kg of body weight. Half of this amount is derived from intracellular fluid and precedes signs of dehydration. The other half is from extracellular fluid and is responsible for signs of dehydration. Typical overall electrolyte loss: 200-500 mEq/L of potassium 300-700 mEq/L of sodium 350-500 mEq/L of chloride. The combination of serum hyperosmolarity, dehydration, and acidosis result in increased osmolarity in brain cells that clinically manifests as an alteration in the level of consciousness. The most common causes for diabetic ketoacidosis (DKA) are: Underlying or concomitant infection (40%) Missed insulin treatments (25%) Newly diagnosed, previously unknown diabetes (15%) Other causes make up roughly 20% in the various scenarios. Initial presentation of type 1 diabetes (25% of patients) Poor compliance with insulin Omission of insulin injections Lack of patient/guardian education Result of psychological stress, particularly in adolescents Bacterial infection and intercurrent illness Brittle diabetes Insulin infusion catheter blockage Mechanical failure of the insulin infusion pump Idiopathic Intercurrent illness Myocardial infarction Pneumonia Prostatitis UTI Medications Corticosteroids Pentamidine, clozapine DKA also occurs in pregnant women, either with preexisting diabetes or with diabetes diagnosed during pregnancy. Physiologic changes unique to pregnancy provide a background for the development of DKA. DKA in pregnancy is a medical emergency, as mother and fetus are at risk for morbidity and mortality. Many of the underlying pathophysiologic disturbances in DKA are directly measurable and need to be monitored throughout the course of treatment. Close attention to clinical laboratory data allows for management of the underlying acidosis and hyperglycemia This can prevent common, potentially lethal complications such as hypoglycemia, hyponatremia, and hypokalemia. Diabetic ketoacidosis Blood glucose over 300 mg/dL Bicarbonate level less than 15 mEq/L pH less than 7.30 ketonemia and ketonuria Severe DKA pH less than 7.1 Bicarbonate less than 5 mEq/L. Serial laboratory tests are critical, including potassium, glucose, electrolytes, and, if necessary, phosphorus. Initial workup should include aggressive volume, glucose, and electrolyte management. Considerations High serum glucose levels may lead to dilutional hyponatremia. High triglyceride levels may lead to factitious low glucose levels. High levels of ketone bodies may lead to factitious elevation of creatinine levels. Extracellular shift of potassium leads to normal or elevated serum potassium, despite severely depleted total body potassium. ICU or monitored bed IVF NS TRA 1000cc/hr for total 20cc/kg Insulin Drip at 0.1 cc/kg/hr Blood tests for glucose every 1-2 h until patient is stable, then every 6 h Serum electrolyte determinations every 1-2 h until patient is stable, then every 4-6 h Initial arterial blood gas (ABG) measurements, followed with bicarbonate as necessary Blood beta-hydroxybutyrate levels measured by a reagent strip and serum ketone levels assessed by the nitroprusside reaction are equally effective in diagnosing DKA in uncomplicated cases. The Acetest and Ketostix products measure blood and urine acetone and acetoacetic acid. They do not measure the more common ketone body, beta-hydroxybutyrate The patient may have paradoxical worsening as the latter is converted into the former during treatment. Specific testing for beta-hydroxybutyrate can be performed by many laboratories. Diagnosis of ketonuria requires adequate renal function. Ketonuria may last longer than the underlying tissue acidosis. One study suggests that routine urine testing for ketones is no longer necessary to diagnose DKA. Using capillary beta hydroxybutyrate offers a distinct advantage of avoiding unnecessary work-up. Beta-hydroxybutyrate levels greater than 0.5 mmol/L are considered abnormal, and levels of 3 mmol/L correlate with the need for treatment for DKA. According to the 2011 Joint British Diabetes Societies (JBDS) guideline for the management of diabetic ketoacidosis: Capillary blood ketones should be measured in order to monitor the response to DKA treatment. The method of choice is bedside measurement of blood ketones using a ketone meter. In the absence of blood ketone measurement, venous pH and bicarbonate should be used together with bedside blood glucose monitoring to evaluate treatment response In patients with DKA, arterial blood gases (ABGs) frequently show typical manifestations of metabolic acidosis, low bicarbonate, and low pH (< 7.2). Venous pH may be used for repeat pH measurements. Brandenburg and Dire found that pH on venous blood gas in patients with DKA was 0.03 lower than pH on ABG. Because this difference is relatively reliable and not of clinical significance, there is almost no reason to perform the more painful ABG. End tidal CO2 has been reported as a way to assess acidosis as well. Serum potassium levels initially are high or within the reference range in patients with DKA. This is due to the extracellular shift of potassium in spite of severely depleted total body potassium. This needs to be checked frequently, as values drop very rapidly with treatment. An ECG may be used to assess the cardiac effects of extremes in potassium levels. The serum sodium level usually is low in affected patients. The osmotic effect of hyperglycemia moves extravascular water to the intravascular space. For each 100 mg/dL of glucose over 100 mg/dL, the serum sodium level is lowered by approximately 1.6 mEq/L. When glucose levels fall, the serum sodium level rises by a corresponding amount. Additionally, serum chloride levels and phosphorus levels always are low in these patients. If the potassium level is greater than 6 mEq/L, do not administer potassium supplement. If the potassium level is 4.5-6 mEq/L, administer 10 mEq/h of potassium chloride. If the potassium level is 3-4.5 mEq/L, administer 20 mEq/h of potassium chloride. Monitor serum potassium levels hourly, and the infusion must be stopped if the potassium level is greater than 5 mEq/L. The monitoring of serum potassium must continue even after potassium infusion is stopped in the case of (expected) recurrence of hypokalemia. In severe hypokalemia, not starting insulin therapy is advisable unless potassium replacement is under way; this is to avert potentially serious cardiac dysrhythmia that may result from hypokalemia. Potassium replacement should be started with initial fluid replacement if potassium levels are normal or low. Add 20-40 mEq/L of potassium chloride to each liter of fluid once the potassium level is less than 5.5 mEq/L. Potassium can be given as follows: two thirds as KCl, one third as KPO4. Even in the absence of infection, the CBC count shows an increased white blood cell (WBC) count in patients with diabetic ketoacidosis. High WBC counts (>15 X 109/L) or marked left shift may suggest underlying infection. BUN frequently is increased in patients with diabetic ketoacidosis. Plasma osmolarity usually is increased (>290 mOsm/L) in patients with diabetic ketoacidosis. If plasma osmolarity cannot be measured directly, it may be calculated with the following formula: plasma osmolarity = 2 (Na + K) + BUN/3 + glucose/18. Urine osmolarity also is increased in affected patients. Patients with diabetic ketoacidosis who are in a coma typically have osmolalities greater than 330 mOsm/kg H2 O. If the osmolality is less than this in a patient who is comatose, search for another cause. Urine and blood culture findings help to identify any possible infecting organisms in patients with diabetic ketoacidosis. Elevated amylase may be seen in patients with diabetic ketoacidosis, even in the absence of pancreatitis. If the patient is at risk for hypophosphatemia (eg, poor nutritional status, chronic alcoholism), then the serum phosphorous level should be determined. Chest radiography should be used to rule out pulmonary infection such as pneumonia. An MRI is helpful in detecting early cerebral edema; it should be ordered only if altered consciousness is present.[ The threshold should be low for obtaining a head CT scan in children with diabetic ketoacidosis who have altered mental status, as this may be caused by cerebral edema. Many of the changes may be seen late on head imaging and should not delay administration of hypertonic saline or mannitol in those pediatric cases where cerebral edema is suspected. DKA may be precipitated by a cardiac event, and the physiological disturbances of DKA may cause cardiac complications. An ECG should be performed every 6 hours during the first day, unless the patient is monitored. An ECG may reveal signs of acute myocardial infarction that could be painless in patients with diabetes, particularly in those with autonomic neuropathy. An ECG is also a rapid way to assess significant hypokalemia or hyperkalemia. T-wave changes may produce the first warning sign of disturbed serum potassium levels. Low T wave and apparent U wave always signify hypokalemia, while peaked T wave is observed in hyperkalemia. Cerebral edema is a serious, major complication that may evolve at any time during treatment of DKA and primarily affects children. It is the leading cause of DKA mortality in children. Deterioration of the level of consciousness in spite of improved metabolic state usually indicates the occurrence of cerebral edema. MRI usually is used to confirm the diagnosis. Cerebral edema that occurs at initiation of therapy tends to worsen during the course of treatment. Mannitol or hypertonic saline should be available if cerebral edema is suspected. 0.5-1 g/kg intravenous mannitol may be given over the course of 20 minutes and repeated if no response is seen in 30-120 minutes. If no response to mannitol occurs, hypertonic saline (3%) may be given at 5-10 mg/kg over the course of 30 minutes. Clinical cerebral edema is rare and carries the highest mortality rate. Although mannitol and dexamethasone (2-4 mg q6-12h) frequently are used in this situation, no specific medication has proven useful in such instances. Recent research by Glaser et al indicated that cerebral edema occurs in 1% of children with DKA, with a mortality rate of 21% and neurologic sequelae in another 21% of patients. Cardiac dysrhythmia may occur secondary to severe hypokalemia and/or acidosis either initially or as a result of therapy in patients with DKA. Usually, correction of the cause is sufficient to treat cardiac dysrhythmia, but if it persists, consultation with a cardiologist is mandatory. Performing cardiac monitoring on patients with DKA during correction of electrolytes always is advisable. Pulmonary edema may occur for the same reasons as cerebral edema in patients with diabetic ketoacidosis. Be cautious of possible overcorrection of fluid loss, though it occurs only rarely. Although initial aggressive fluid replacement is necessary in all patients, particular care must be taken in those with comorbidities such as renal failure or congestive heart failure. Diuretics and oxygen therapy often suffice for the management of pulmonary edema. Nonspecific myocardial injury may occur in severe DKA, which is associated with minute elevations of myocardial biomarkers (troponin T and CK-MB) and initial ECG changes compatible with myocardial infarction (MI). Acidosis and very high levels of free fatty acids could cause membrane instability and biomarker leakage. Coronary arteriography usually is normal, and patients tend to recover fully without further evidence of ischemic heart disease. The presence of minute biomarker elevations and ECG changes do not necessarily signify MI in DKA. Hypoglycemia In patients with diabetic ketoacidosis, hypoglycemia may result from inadequate monitoring of glucose levels during insulin therapy. Hypokalemia Hypokalemia is a complication that is precipitated by failing to rapidly address the total body potassium deficit brought out by rehydration and insulin treatment, which not only reduces acidosis but directly facilitates potassium reentry into the cell. Managing DKA in the ICU during the first 24-48 hours always is advisable. When treating patients with DKA, the following points must be considered and closely monitored: Correction of fluid loss with intravenous fluids Correction of hyperglycemia with insulin Correction of electrolyte disturbances, particularly potassium loss Correction of acid-base balance Treatment of concurrent infection, if present It is essential to maintain extreme vigilance for any concomitant process, such as infection, cerebrovascular accident, myocardial infarction, sepsis, or deep venous thrombosis. When the blood glucose falls below 14 mmol/l (250 mg/dl), 10% glucose should be added to allow the fixed-rate insulin to be continued. If already taking, long-acting insulin analogues such as insulin glargine (Lantus) should be continued in usual doses. The diabetes specialist team should be involved as soon as possible Once the patient is stable, transfer to regular hospital bed. If new onset DM, calculate daily insulin dose 0.3 units/kg/day for patients who are lean, on hemodialysis, frail and elderly, insulin-sensitive, or at risk for hypoglycemia 0.4 units/kg/day for a patient at normal weight 0.5 units/kg/day for overweight patients 0.6 units/kg/day or more for patients who are obese, on high-dose steroids or insulin-resistant Between 40% and 50% of that total dose should be administered as basal, using Lantus or other long-acting insulin. The remainder is divided to be given as shortacting insulin (Humalog) prior to each meal. Check sugars in the AM fasting and 2 hours after each meal to evaluate the regimen. The overall mortality rate for DKA is 2% or less. The presence of deep coma at the time of diagnosis, hypothermia, and oliguria are signs of poor prognosis. The prognosis of properly treated patients with diabetic ketoacidosis is excellent, especially in younger patients if intercurrent infections are absent. The worst prognosis usually is observed in older patients with severe intercurrent illnesses (eg, myocardial infarction, sepsis, or pneumonia), especially when these patients are treated outside an intensive care unit. When DKA is treated properly, it rarely produces residual effects. With modern fluid management, the mortality rate of DKA is about 2% per episode. Before the discovery of insulin in 1922, the mortality rate was 100%. In the last 20 years, mortality rates from DKA have markedly decreased, from 7.96% to 0.67%.[10] DKA accounts for 14% of all hospital admissions of patients with diabetes and 16% of all diabetes-related fatalities. A fetal mortality rate as high as 30% is associated with DKA. The rate is as high as 60% in diabetic ketoacidosis with coma. Fetal death typically occurs in women with overt diabetes, but it may occur with gestational diabetes. In children younger than 10 years, diabetic ketoacidosis causes 70% of diabetes-related fatalities. Regular follow up Management of risk factors Patient/parent education