to this slideshow
advertisement

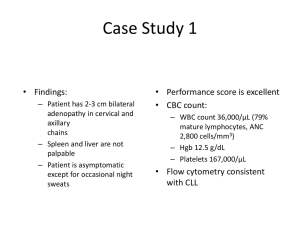
Rheumatoid Arthritis: Expanding Treatment Options HIGHLIGHTS FROM EULAR 2008 PRESENTATIONS June 11-14, 2008 Paris, France Rituximab is Approved After Failure with 1 anti-TNFa Inhibitor • Is there a benefit to using Rituximab, instead of another TNFa-inhibitor, when a patient has already failed a TNFa-inhibitor? • What are long-term results of its use in the real World? • What are the options when Rituximab doesn’t work? • Can you use a TNFa-inhibitor with Rituximab? Switching to rituximab vs. alternative second or third TNF-inhibitor after treatment failure to first TNF-inhibitor Rationale • About 30% of patients on anti-TNF agents do not respond to treatment and large numbers of responders eventually lose their response • When patients fail one anti-TNF agent, switching to another class of agent such as rituximab may be more effective than opting for a second or third TNF inhibitor Finckh et al. EULAR 2008, abstract OP-0249. Decreased effectiveness to a Second anti-TNF EULAR response rates 3 months after anti-TNF initiation Adapted from Navarro et al. Arthritis Rheum 2006;54:abstract 873 (Spanish RA cohort) Switching to Rituximab vs. alternative second or third TNF-inhibitor after treatment failure to First TNF-inhibitor Patients and treatment 300 patients had failed at least one anti-TNF agent • 101 patients received a first cycle of rituximab • 199 an alternative anti-TNF agent Primary outcome: Evolution of DAS28 scores over the 1st year Finckh et al. EULAR 2008, abstract OP-0249. Initiate treatment with a biological agent with a different mechanism of action: Lasting change in DAS28 over 24 weeks with rituximab Mean change in DAS28 Weeks In RA patients with an inadequate response to anti-TNFs, the REFLEX trial has demonstrated that rituximab is more effective than placebo Adapted from Cohen et al. Arthritis Rheum 2006; 54: 2793–806 Results • Overall evolution of DAS28 was more favourable in the rituximab group than in the anti-TNF group (P=0.01) DDAS28 score after six months according to reason for switching Rituximab Ineffectiveness to previous antiTNF agent -1.55 -1.03 Significant Adverse event -0.86 -0.77 Non-significant Alternate anti-TNF This study suggests that rituximab is more effective than switching to an alternative anti-TNF agent in patients who have persistent active disease despite anti-TNF therapy Finckh et al. EULAR 2008, abstract OP-0249. The STURE Registry (Stockholm TNFa uppföljningsergister) • The STURE registry is a comprehensive registry in the larger Stockholm area of patients receiving biological therapies for RA • The registry described experience with rituximab in 114 of these patients • EULAR disease activity: 78% high, 19% moderate Baseline demographics Female 83% Age 57 ± 14 Female; 58 ± 11 Male Disease duration (years) 12 ± 14 Rheumatoid Factor (RF)-positive antibodies 91% Number of prior anti-TNF 2.01 ± 0.81 DAS28 6.03 ± 1.26 (Female: 5.96 ±1.22; male: 6.42 ±1.41) HAQ disability index 1.57 ±0.59 van Vollenhoven et al. EULAR 2008, abstract FRI0168. (Female: 1.64 ±0.58; male: 1.21 ±0.54, P=0.01) The STURE Registry: DAS28 Response P=0.002 P<0.001 van Vollenhoven et al. EULAR 2008, abstract FRI0168. The STURE Registry EULAR Response n=71 n=51 n=27 • At 3 months 64% of patients achieved a good/moderate response; 19% a good response and 14% a DAS28 <2.6 • At 6 months, 62% achieved a good/moderate response; 23% a good response and 18% a DAS28 <2.6 van Vollenhoven et al. EULAR 2008, abstract FRI0168. The STURE Registry Conclusions • After 9 months of follow-up, there was no difference in efficacy between patients treated with or without MTX • Efficacy appears similar in RF+ and RF– patients • Results in this registry were consistent with clinical trials • Rituximab is a useful therapy in patients with very active, TNF-refractory RA, with 60-70% having a EULAR response and 20-30% achieving low disease activity or remission van Vollenhoven et al. EULAR 2008, abstract FRI0168. A UK Single-centre Experience • Real-world” clinical experience with any drug does not always reflect that of clinical trials • It is important to understand if general use of rituximab offers patients similar efficacy and safety as reported in clinical trials • Investigators describe a single-centre experience in which the long-term effects of rituximab were documented for their first 100 patients Characteristics for all patients: • DAS28 >5.1 • RF-positive or anti-CCP antibody • Inadequate response or contraindication to anti-TNF agents • Clinical outcome was determined at 6 months by EULAR criteria Dass et al. EULAR 2008, abstract AB0352 Mean DAS 28 scores at 6 months improved with each cycle A UK Single-centre Experience • Mean DAS28 scores at 6 months improved with each cycle • Only 6 patients out of the 100 treated did not respond to Rituximab therapy (94% response rate) • All good responses were maintained after the second cycle and 18% of previously moderate responders improved further and achieved good responses • Initial non-response does not preclude response to further therapy Dass et al. EULAR 2008, abstract AB0352 A UK Single-centre, Real-world Experience • Rituximab appears to be well tolerated, even in those patients with complex disease • 19% were on leflunomide instead of methotrexate, 6% were not on a DMARD – No differences in duration of response observed in any of these groups Dass et al. EULAR 2008, abstract AB0352 A Real-world Experience in a Canadian Centre • Investigators reviewed the use of rituximab in severe RA patients refractory to DMARD and anti-TNF therapy • A total of 21 patients from a prospective longitudinal database in Calgary were included Hazlewood et al. EULAR 2008, abstract AB0362 A Real-world experience in a Canadian Centre Assessment at 3 months Good or moderate EULAR response Average DAS28 score Average mHAQ 82% -2.6 -0.63 Eight patients required a second course of rituximab after an average of 10.4 months, and one required a third course • 2 cases of serious infections and 4 of non-serious infections • Three infusion reactions occurred in 2 patients • Nonspecific reactions occurred in 3 patients Hazlewood et al. EULAR 2008, abstract AB0362 A Real-world Experience in a Canadian Centre Conclusions • Rituximab is efficacious in the treatment of patients with severe RA who are intolerant or resistant to anti-TNF therapy • Results support evidence that depletion of B cells with rituximab is efficacious in RA • Adverse events did occur but none were fatal Hazlewood et al. EULAR 2008, abstract AB0362 Optimizing response to retreatment with rituximab • It was previously reported that for every 1.0 point the DAS28 was allowed to worsen before a 2nd course of rituximab, patients had a 0.32 point higher DAS28 postretreatment • Investigators explored whether the more clinically accessible Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI) might also be predictive of retreatment outcome • Analysis was based on the REFLEX trial (Arthritis & Rheumatism 2006;54(9):2793-806) and subsequent open-label extension study Mease et al. EULAR 2008, abstract THU0188 REFLEX Study design and open-label extension phase Screen/TNF and/or DMARD withdrawal period Open-label Extension study Double-blind course 1 Randomization • • • • • • Rituximab + MTX (Group A)* Methotrexate (MTX) x ≥ 3 months Placebo + MTX (Group B)* Screen Day 1 Week Week Week Week Week Week Week 2 4 8 12 16 20 24 Rituximab: 1000 mg or placebo iv infusion Methylprednisolone: 100 mg iv before rituximab infusion Prednisone: 60mg day 2-7, 30mg day 8-14 • Clinic visit Rescue Standard of care Primary efficacy time point Rituximab +MTX *Note: The group A and B ITT populations were 298 and 201, respectively. Mease et al. EULAR 2008, abstract THU0188 Timing of course 2 was at physician discretion SDAI and CDAI for Predicting Outcome of a Second Course of Rituximab for Patients with RA Mean disease activity scores by time to course 2 intervals BSE (Day 1C1) Week 24 C1 Prior to C2 Week 24 C2 ≤36 weeks 50.00 30.26 37.27 23.71 37-52 weeks 51.48 17.23 43.09 15.75 >52 weeks 47.55 16.35 39.04 18.36 ≤36 weeks 46.25 28.11 34.96 23.17 37-52 weeks 47.31 15.66 39.76 14.98 >52 weeks 44.08 15.20 37.15 17.06 SDAI CDAI Mease et al. EULAR 2008, abstract THU0188 Percentage change from baseline for A) SDAI, B) CDAI and C) DAS28 Mease et al. EULAR 2008, abstract THU0188 SDAI and CDAI for Predicting Outcome of a Second Course of Rituximab for Patients with RA • Every 1.0 point that SDAI was allowed to worsen before C2 resulted in a 0.21 point higher SDAI score post-C2 • Every 1.0 point that CDAI was allowed to worsen before C2 resulted in a 0.24 point higher CDAI score post-C2 • These data suggest that outcome following a second course of rituximab is better the less that disease activity is allowed to worsen before repeating treatment • Retreatment before patients are allowed to flare results in improved disease activity after C2 rituximab Mease et al. EULAR 2008, abstract THU0188 Early use of rituximab in patients with severe RA refractory to DMARDS Patient Characteristics 13 women and 2 men with severe RA and poor response to conventional therapy with multiple DMARDS, corticosteroids and NSAIDs. The average age was 52 years old with an average disease duration of 3 years. Marked extra-articular manifestations RF-positive at high titers DAS28 >5.6 HAQ = 1.4 No prior use of anti-TNF therapy Guzman et al. EULAR 2008, abstract AB0358 Results and Conclusion 80% of improvement in clinical parameters was observed at an average of three weeks after initiation of treatment. Response ACR20 ACR50 ACR70 Week 24 62% 42% 21% Average HAQ score remained low at 0.5 Median improvement in DAS28 score -2.4 in 13 patients No serious adverse events reported at 2 years follow-up Rituximab may be an excellent alternative as a first-line biological agent in patients with aggressive disease Guzman et al. EULAR 2008, abstract AB0358 Rituximab ACR and DAS28 response after 24 weeks in RA patients with inadequate response to one TNFa inhibitor: The RESET trial An open-label, single-arm, multicentre, international study (26 sites in Canada, 9 in Sweden) of safety and efficacy of a combination of rituximab with methotrexate in patients with active RA with an inadequate response or intolerance to only one prior TNF-inhibitor Interim analysis in 50 patients with a follow-up of 24 weeks after 2 infusions of rituximab 1000 mg Mean DAS28 at baseline 6.4 ±1.2 Haraoui et al. EULAR 2008, abstract AB0360 Results Response at Week 24 ACR20 ACR50 ACR70 60% 28% 8% Baseline Week 24 Swollen joint count 13.7 6.8 Tender joint count 15.5 6.2 Patient global 67.3 36.4 Physician global 65.5 28.9 HAQ 1.8 1.3 CRP mg/dL 3.0 1.2 ESR mm/hr 39.0 20.4 Haraoui et al. EULAR 2008, abstract AB0360 Results • FACIT-F scores decreased by 34% by week 24 • EULAR good/moderate response at 4, 12, and 24 weeks were 54%, 74% and 78%, respectively • DAS remission was seen in 8% of patients and low disease activity in 20% by week 24 • Mean DDAS28 of -2.2 at Week 24 from a baseline of 6.4 ±1.2 Haraoui et al. EULAR 2008, abstract AB0360 Conclusions • A single course of rituximab with MTX provided clinically significant improvements in disease activity by week 24 in patients with active, long-standing RA who had an inadequate response to one prior anti-TNF agent • Small improvements in HAQ are probably due to irreversible damage seen in a cohort with long-standing disease Haraoui et al. EULAR 2008, abstract AB0360 Combination therapy with rituximab and etanercept for patients with RA • Six patients with long-standing refractory RA who failed rituximab were treated with etanercept two months after receiving rituximab • Over 6 months, DAS28 significantly decreased to 4.2 and high serum CRP levels of 68.9 mg/L prior to receiving the combination decreased to 7.4 mg/L • No severe infections occurred over a mean of 10.7 months and the frequency of mild bronchitis or influenza was not increased with the combination • Short-term results are promising but without long-term experience, the rituximab/etanercept combination cannot yet be recommended Blank et al. EULAR 2008, abstract THU0165. Safety and efficacy of rituximab in patients with chronic infection • Five patients with active chronic infections not eligible for antiTNF therapy were treated with rituximab. • Mean treatment duration: 15.6 months • ACR20 at six months was 59% and 45% at 12 months. • Patients did not experience any adverse events or worsening of their chronic infection, suggesting that rituximab may be a safe and effective therapy in patients with chronic infections in whom anti-TNF therapy is contraindicated Continuous courses of rituximab do not increase the frequency of severe infections and for patients with contraindications to anti-TNF agents, B-cell depletion may be an attractive alternative Casas et al. EULAR 2008, abstract AB0378 Summary of Findings •Rituximab may more effective than switching to an alternative antiTNF agent in patients with persistent active disease •Rituximab is a useful therapy in patients with very active, TNFrefractory RA and efficacy appears similar in RF+ and RF– patients •Duration of response with Rituximab is at least 6 months •Subsequent courses of Rituximab continue top provide added benefit •Rituximab appears to be well tolerated even by patients with complex disease •Continuous courses of rituximab do not increase the frequency of serious infections •Rituximab is an excellent alternative as a first-line biological agent in patients with aggressive disease