Breast Cancer - Scientific Organizing Service
advertisement

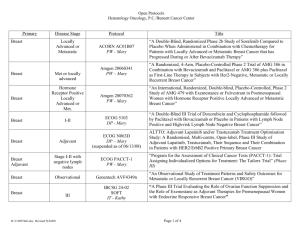
Take home message Breast Cancer Bevacizumab in MBC Sabino De Placido 1 Survival of Patients with Metastatic Breast Cancer 1974 - 2000 1.0 30 .8 No. Drugs Available 25 1995-2000 1990-1994 .6 1985-1989 .4 1980-1984 20 15 10 5 .2 1974-1979 0 1950s 1960s 1970s 1980s 1990s 0.0 0 12 24 36 Months 48 60 International Guidelines for Management of Metastatic Breast Cancer: Combination vs Sequential Single-Agent Chemotherapy Fatima Cardoso , Philippe L. Bedard , Eric P. Winer , Olivia Pagani , Elzbieta Senkus-Konefka , Lesley J. Fallowfield , Stella Kyriakides , Alberto Costa , Tanja Cufer , Kathy S. Albain ; on behalf of the ESO-MBC Task Force J Natl Cancer Inst 2009;101:1174–1181 In the absence of evidence to guide daily clinical decision making in MBC, both combination and sequential single agent chemotherapy are reasonable options as first-line systemic therapy. An important question for future research is the clear definition of patients who may benefit from a combination approach. Until such data are available, the ESO-MBC Task Force believes that sequential single-agent therapy should be the preferred choice for most MBC patients, in the absence of rapid clinical progression, life-threatening visceral metastases, or the need for rapid symptom and/or disease control. These recommendations reflect consensus expert opinion and represent level 5 clinical evidence. 1/5 Take home message Metastatic Breast Cancer No single «gold standard» in metastatic breast cancer 4 Breast Cancer Bevacizumab in first line MBC 5 Comparison of the Studies (1/2) No. of patients Geography Randomization ratio (BV:PL) Primary Endpoint Independent review E2100 AVADO* RIBBON-1* 722 488 1237 US (90%) Ex-US US (50%) 1:1 1:1 2:1 PFS† PFS PFS Retrospective No Prospective BV=bevacizumab, PL=placebo, PFS=progression-free survival, ORR=objective response rate, OS=overall survival. * Permitted continuing on BV or crossing over to BV. † Analyses based on IRF assessments. Comparison of the Studies (2/2) Placebo controlled Chemotherapy Bevacizumab dose Key Secondary Endpoints E21001 AVADO2 RIBBON-13 No Yes Yes Weekly paclitaxel 3-weekly docetaxel 10 mg/kg q2w 7.5 or 15 mg/kg q3w OS, ORR OS, ORR, 1-yr survival Capecitabine Taxane or anthracycline 15 mg/kg q3w OS, ORR, 1-yr survival 1. Miller, et al. NEJM 2007; 2. Miles, et al. ASCO 2008; 3. Robert, et al. ASCO 2009 2/5 Take home message Metastatic Breast Cancer Study Results Remarkable consistency in all study results 8 Consistent Benefit with Bevacizumab-Based Therapy: Significant Improvement in PFS E2100 AVADO RIBBON-1 (Cape) RIBBON-1 (Tax/Anthra) NonBV BV NonBV BV* NonBV BV NonBV BV Median PFS, mo 5.8 11.3 7.9 8.8 5.7 8.6 8.0 9.2 Stratified HR (95% CI) 0.48 (0.39–0.61) 0.62 (0.48–0.79) 0.69 (0.56–0.84) 0.64 (0.52–0.80) p<0.0001 p=0.0003 p=0.0002 p<0.0001 p-values BV=bevacizumab, Cape=capecitabine, Tax/Anthra=taxane/anthracycline. * 15 mg/kg cohort. Consistent Benefit with Bevacizumab-Based Therapy: Significant Improvement in ORR E2100 ORR (%) p-values AVADO RIBBON-1 (Cape) RIBBON-1 (Tax/Anthra) NonBV BV NonBV BV* NonBV BV NonBV BV 23 41 46 64 23.6 35.4 37.9 51.3 p<0.0001 p=0.0003 p=0.0097 BV=bevacizumab, Cape=capecitabine, Tax/Anthra=taxane/anthracycline. * 15 mg/kg cohort. p<0.0054 No Statistically Significant Difference in OS E2100 Median OS, mo RIBBON-1 (Tax/Anthra) BV NonBV BV* Non-BV BV Non-BV BV 24.8 26.5 31.9 30.2 21.2 29.0 23.8 25.2 p-values p-values RIBBON-1 (Cape) NonBV Stratified HR (95% CI) 1 year rate (%) AVADO 74 0.87 1.03 0.85 1.03 P=0.14 P=0.85 P=0.87 P=0.83 81 P=0.017 76 84 P=0.02 74 81 P=0.076 BV=bevacizumab, Cape=capecitabine, Tax/Anthra=taxane/anthracycline. * 15 mg/kg cohort. 83 81 P=0.44 A Meta-Analysis of Overall Survival Data from Three Trials of Bevacizumab and First-Line Chemotherapy as Treatment for Patients with Metastatic Breast Cancer Joyce O’Shaughnessy, David Miles, Robert Gray, Véronique Diéras, Edith A. Perez, Robin Zon, Javier Cortés, Xian Zhou, See-Chun Phan, Kathy Miller Baylor-Sammons Cancer Center, Texas Oncology, US Oncology, Dallas, TX; Mount Vernon Cancer Centre, London, England; Dana-Farber Cancer Institute, Boston, MA; Institut Curie, Paris, France; Mayo Clinic, Jacksonville, Florida; Michiana Hematology Oncology, South Bend, IN; Vall d'Hebron University Hospital, Barcelona, Spain; BioOncology, Genentech, S San Francisco, CA; Indiana University Melvin and Bren Simon Cancer Center, Indianapolis, IN ASCO, 2010 E2100 Paclitaxel Previously Untreated MBC AVADO Docetaxel RIBBON-1 Capecitabine, Taxane, or Anthracycline RANDOMIZE General Study Designs Chemo + No BV Chemo + BV Treat until PD Optional Second-line Chemo + BV (AVADO and RIBBON-1 only) Progression-Free Survival, Pooled Population Non-BV (n=1008) BV (n=1439) Median, mo 6.7 9.2 HR (95% CI) 0.64 (0.57–0.71) Summary of Pooled Efficacy Analysis • PFS - HR=0.64, 36% reduction in risk of PD or death - 2.5 month improvement in median PFS - Improvements across key clinical subpopulations • ORR - 17% increase vs controls • OS - No statistically significant difference Metastatic Breast Cancer Clinical Relevance 16 Clinical Relevance • Is 2.5 month improvement in median PFS a clinically relevant result ? PFS Bevacizumab + Paclitaxel vs Paclitaxel 2.5 mos Anthracyclines + Taxanes vs Taxanes 0.8 mos Capecitabine + Docetaxel vs Docetaxel 1.9 mos Gemcitabine + Paclitaxel vs Paclitaxel 2.1 mos 3/5 Take home message Metastatic Breast Cancer Clinical Relevance The improvement in PFS is similar to that of most other first line studies 18 Metastatic Breast Cancer Adverse Events 19 E2100, AVADO & RIBBON1 Metanalysis Grade ≥3 Selected Adverse Events (Aes) 20 4/5 Take home message Metastatic Breast Cancer Adverse Events Well tolerated in MBC patients and AE are fairly manageable 21 Metastatic Breast Cancer Improvements across key clinical subpopulations 22 5/5 Take home message Metastatic Breast Cancer Improvements across key clinical subpopulations The advantage may be relevant in triple negative breast cancer 27