Monitoring Adherence to Treatment for Chronic
Diseases ---Using osteoporosis as an example
from Taiwan
Tzu-Chieh Lin1
Prof. Yea-Huei Kao Yang1,2
1Institute of Clinical Pharmacy and Pharmaceutical Sciences,
2Health Outcome Research Center, College of Medicine,
National Cheng Kung University, Tainan, Taiwan
Conflicts of interest
• Our study was supported in part by grants from the
Multidisciplinary Center of Excellence for Clinical Trial
and Research (DOH100-TD-B-111-002)
• Department of Health, Executive Yuan, Taiwan and
National Science Council, Taiwan (NSC 99-2320-B006-016-MY3)
Background - 1
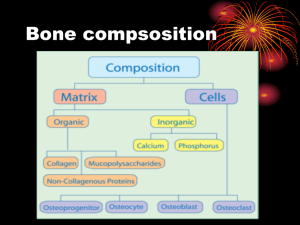
• Osteoporosis is characterized by decreased bone
mass, deterioration of bone tissue and disruption of
bone architecture
– ↓ bone strength , ↑fracture risk
• A major public health burden in developed countries
– 10 million people ≥50 years of age have osteoporosis
in USA →1.5 million fractures annually
• Patients with prior osteoporotic fractures → 2 X
higher risk of future fractures
– Secondary prevention of osteoporotic fractures →
standard practice worldwide
Background - 2
• Bisphosphonates are recommended as the primary
pharmacological therapy for secondary prevention of
osteoporotic hip fractures
– ↓ the risk of hip fractures by 40–50%
• Long-term compliance is necessary to ensure optimal
therapeutic efficacy
– <50% of the patients were compliant during the first year
after initiation of treatment
• Several studies using claims databases have estimated
the impact of compliance on preventing further fracture
events
– 20–60% reduction in overall fracture risk
Background - 3
• The reimbursement scheme of the Bureau of
National Health Insurance in Taiwan
– Osteoporosis drugs → patients who have had
osteoporotic vertebral or hip fractures
– Provides an invaluable opportunity to assess the
impact of compliance on outcome in patients who
have already had osteoporotic fractures
Clin Pharmacol Ther 2011 90(1): 109-116
Significance & Objectives
• Previous studies focusing on treatment compliance
and its impact on fracture risks
– Mainly in the developed countries
• The objectives of the our study
(i) To describe the first-year treatment compliance of
patients initiated on alendronate therapy after
osteoporotic vertebral or hip fractures
(ii) To assess the impact of compliance on the risks of
subsequent hip fracture over a longer period
Method – Data source
• National Health Insurance Research Database (NHIRD)
– Demographic data for enrollees
– Information regarding health-care professionals and
facilities
– Service claims from inpatient, ambulatory care, and
contracted pharmacies
Method – Study design and Population
• A retrospective cohort analysis, 2003-2006
– Patients >50 years of age with new osteoporotic vertebral
or hip fractures and new to alendronate therapy
• The index date → the first day on which patients
received an alendronate prescription
– The baseline period was defined as the year preceding the
index date
• To ensure that the index fracture was related to
osteoporosis
– Patients had at least one osteoporosis-related claim during
the baseline period
Method – Study Population
• Exclusion criteria
– Patients who had experienced any prior osteoporotic
vertebral/hip fracture
– Patients whose index osteoporotic fracture was associated
with car accidents or high-impact trauma
– Diagnosis of Paget’s disease or malignant neoplasm
• Follow-up period
– Compliance with alendronate → The 1st year
– Impact of compliance on fracture risk → From the index
date to the first date of an incident hip fracture or to the
end of the study
Method - Compliance with
alendronate treatment
• Alendronate is currently the only oral
bisphosphonate that approved for insurance
reimbursement for osteoporotic fracture
• Refill compliance was defined as the medication
possession ratio (MPR) for the follow-up period
– Dividing the total number of defined daily doses the
patient received by the follow-up period
– MPR ≥80% as good compliance
– Examined the results by adjusting the cutoff point
upward and downward
Outcome and Covariates
• Incident hip fracture
– Retrieved from inpatient claims only
• Demographic characteristics (age, gender)
• Index osteoporotic fracture, presence of kyphosis, history of any
other fracture (radius/ulna, humerus, and other nonvertebral
fractures except hip fracture)
• Comorbid conditions that could increase fracture risk (Alzhelmer’s
disease, asthma, diabetes mellitus, ischemic stroke, history of falls,
and rheumatic arthritis)
• Comedications (antiepileptics, β-blockers, benzodiazepines,
glucocorticoids, hormone replacement therapy, COX-2 agents,
selective serotonin reuptake inhibitors, thyroid drugs, and
sleep/hypnotic agents).
Statistical analysis
• Student’s t-test or χ2 → Primary analysis
• Time-to-event analysis → Impact of compliance
– A time-dependent covariate for compliance
– Multivariate Cox proportional hazard models with time-dependent
covariates
– Determined whether covariates fitted a proportional hazards
assumption
• Sensitivity analyses
– Different thresholds of good compliance, MPR as a continuous variable
– Female patients only, types of index osteoporotic fracture, patients
with/without any other fracture 1 year before treatment initiation,
stratified patient age groups with 65 years as a cutoff point, and
patients not on hormone replacement therapy
– Excluding the data for patients who had an incident hip-fracture event
within 6 months after treatment initiation
Discussion
• This retrospective analysis of Taiwanese patients with
osteoporotic vertebral or hip fractures who were
new to alendronate found :
– Only 38% of patients to be compliant during the first
year
– Compliant patients had significantly lower hip-fracture
risk as compared with noncompliant patients
– The results were consistent through various sensitivity
analyses
Discussion
• It is difficult to make a direct comparison of
compliance rates among published studies because
of their use of different covariates for adjustment
– Age, sex, fracture history, and medications of interest
• Several studies have used claims databasesto assess
patients’ compliance
– MPR: 61-74% in the States, Canada or UK
– In our study: 60.2% in Taiwan
Discussion – Impact of compliance
ETHEL S. SIRIS et al, 2006
Cohort
Study type
Medstat MarketScan
Database
Arlene M Gallagher et al. 2008 V. Rabenda et al,
2008
Cohort
GPRD
Commercial Claims and
Encounters and Medicare
databases
Patients ≥45 yr with claims Women or men ≥18 yr of age who
Patient
for BP
received a prescription for
population
alendronate or risedronate.
Prevention
Fracture
type
ART
Primary
Traditional osteoporotic fracture
sites (vertebrae, humerus,
radius, ulna, clavicle, pelvis,
femoral
neck, and femur), as well as the
patella, tibia, fibula, and ankle
Alendronate or Risedronate
2 year
Follow-up
period
0.557
Impact of
compliance
Reference Mayo Clin Proc.
2006;81(8):1013-1022
Primary
Osteoporotic fracture (defined as a
hip/femur, vertebral, radius/ulna,
humerus, rib, or pelvis fracture),
hip/femur fracture, radius/ulna
fracture
Alendronate or Risedronate
Our study
Cohort
The Belgian national
database
Cohort
NHIRD
Postmenopausal
women aged>45 years,
and were new users
with previous vertebral
fractures
Secondary
Postmenopausal
women aged
above 50yrs, with
prior vertebral or
hip fracture
Secondary
Hip fracture
Hip
Alendronate
Alendronate
1 year
At least year
4 year
0.78
0.40
0.30
Journal of Bone & Mineral
Research 23(10): 1569-1575
Osteoporosis
International 19(6): 811818
Discussion – Sensitivity analysis
• Most studies using MPR ≥80% as the threshold for good
compliance
– We varied the threshold for good compliance in steps from
70 to 100%
• The benefit of compliance was pronounced even when
the alendronate treatment was for secondary prevention
– Adjusted HR, 0.28; 95% CI 0.18–0.51
• The most pronounced reduction in patients with no
history of fracture prior to the index osteoporotic
fracture
Discussion - Strength
• The first large-scale one in Asia to assess the association
between treatment compliance and fracture risk
• Demonstrated a pronounced benefit of compliance in
preventing secondary hip fracture
• The duration of follow-up
– Most published compliance studies → 1–2 years
– Up to 4 years in our study
• Included various covariates
– Age, comorbidities, and co-medications that were thought
to be related to osteoporotic fractures
Discussion - Limitations
• The inherent weakness of an observational study and the
administrative database → residual confounders
– Lack of socioeconomic covariates → confounding by
lifestyle
– Body mass index, smoking status, and caffeine intake
• Misclassification of compliance
– Comprehensively captured prescription claims from
inpatient, outpatient, and contracted pharmacies
• Patients who received HRT may have benefited from its
protective effect
– Consistent results were found even after excluding data for
those kinds of patients
Discussion – Clinical implications
• The main policy of Taiwan’s Bureau of National
Health Insurance regarding osteoporotic fractures
was secondary prevention
– Fracture sites other than vertebra/hip (e.g., radius and
ulna) ↑ 2 X incident hip-fracture risk
– Higher fracture risk in older patients
Summary
• The compliance status among Taiwanese osteoporotic
patients new to alendronate was suboptimal within the
first year after treatment initiation
• Compliant patients had a significantly lower incident hipfracture risk as compared with noncompliant patients
• In real-world setting → osteoporosis drugs will not work
optimally unless patients actually take them
– Every effort should be made to gain greater insight into the
factors associated with poor compliance and to initiate
interventions to improve patient adherence.
Thanks for your attention!
tb897104@mail.ncku.edu.tw