Vestibular and Auditory Ototoxicity
advertisement

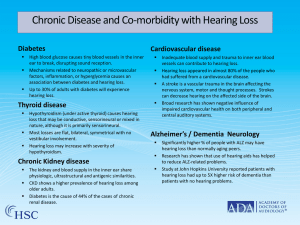
Dr Mostafa Hashemi 2 Ototoxicity refers to the tendency of a drug or chemical agent to cause inner ear dysfunction,or Inner ear tissues damage either temporarily or permanently and induce symptoms of hearing loss, dizziness, or both. 3 Aminoglycoside Antibiotics used to fight gram negative bacilli and staphylococci Some Anti‐Neoplastics Used to treat head and neck, lung, gynecologic and testicular cancers Loop Diuretics Used to treat congestive heart failure, renal failure, cirrhosis and hypertension Quinines Used to treat malaria and occasionally for nocturnal leg cramps Salicylates Used in treatment of TIAs, stroke, unstable angina and myocardial infarction 4 The aminoglycoside antibiotics are an important class of anti-infectious agents. They were developed to combat tuberculosis and other life-threatening infections. The first members of this class of drugs were streptomycin and dihydrostreptomycin. Initial clinical trials showed that these compounds could damage the kidneys and the inner ear. Since then, many new aminoglycosides have been developed. 5 Neomycin was found to be too toxic for systemic use and has been relegated to local application. Other members of this group of drugs include kanamycin, gentamicin, tobramycin, amikacin, netilmicin, and sisomicin. Some of these agents are more toxic to either the cochlea or the vestibular apparatus, although their ototoxicity is not completely selective 6 Toxicity generally occurs only after days or weeks of exposure. The overall incidence of aminoglycoside auditory toxicity is estimated to be approximately 20%, whereas vestibulotoxicity may occur in about 15%. 7 Aminoglycosides are excreted primarily by the kidney by glomerular filtration. Impaired renal function reduces the rate of excretion. Renal failure is a risk factor for ototoxicity, and dosing of aminoglycosides must be modified to compensate for delayed renal excretion. Measurement of peak and trough serum levels of aminoglycosides provides rough guidelines for therapeutic efficacy, but is not an absolute guarantee for prevention of ototoxicity, particularly vestibular ototoxicity. 8 Aminoglycoside ototoxicity may be detected, but because of a life-threatening infection and a lack of suitable alternative antibiotic therapy, it may be necessary to continue treatment Antibiotic ototoxicity may continue even after cessation of aminoglycoside therapy. Aminoglycosides trigger apoptosis at clinically relevant doses, whereas higher doses may trigger necrotic cell death. 9 Animal and human temporal bone histopathologic studies show that the cochlear and vestibular hair cells serve as primary targets for injury. In the organ of Corti, the outer hair cells of the basal turn are damaged first. As drug treatment is continued, the damage may spread to more apical regions. Inner hair cells seem to be more resistant to injury than outer hair cells. This difference could be a result of the higher concentration of the natural antioxidant, glutathione, in the inner hair cells and in the apical turn outer hair cells compared with in the outer hair cells of the basal turn.. 10 High-frequency hearing loss tends to occur first and may be detectable before it becomes clinically noticeable. [15] Continued exposure to aminoglycosides may result in hearing loss that progresses to lower frequencies, including the important speech range, interfering with communication skills. A hearing loss of 20 dB or more at two or more adjacent frequencies should be documented to confirm the diagnosis of drug related hearing loss after exclusion of other causes of hearing loss. Delayed ototoxicity may occur after cessation of treatment with aminoglycoside antibiotics. Delayed onset of hearing loss usually manifests within 1 to 3 weeks after the end of therapy. [16] 11 Rotational vestibular testing in patients receiving aminoglycoside therapy who were asymptomatic is normal When responses to all frequencies were absent, oscillopsia and severe imbalance were usually present. 12 1- Bacteremia, fever, hepatic dysfunction, and renal dysfunction have been associated with ototoxicity 2- Combinations of another ototoxic drug and an aminoglycoside may increase the risk and severity of ototoxicity 13 14 15 Cisplatin is a potent antineoplastic agent that is used to treat various malignant tumors, including ovarian,testicular, bladder, lung, and head and neck carcinomas. Side effects include nausea and vomiting,neurotoxicity, ototoxicity, and nephrotoxicity. 16 Hearing loss caused by cisplatin seems to be highly variable, and seems to be related to dose; age of the patient; and other factors such as noise exposure, exposure to other ototoxic drugs, depleted nutritional state including low serum albumin and anemia, and cranial irradiation. Children seem to be more susceptible than adults. Hearing loss tends to be permanent and bilaterally symmetric. 17 Symptoms of ototoxicity include subjective hearing loss, ear pain, and tinnitus. Tinnitus has been reported in 2% to 36% of patients treated with cisplatin, and may be transient or permanent. 18 Although higher frequencies are affected first, hearing impairment may extend into the middle frequency range when doses greater than 100 mg/m 2 are used. When ultra-high-frequency audiometric testing is used, 100% of patients receiving high-dose cisplatin (150 to 225 mg/m 2 ) may show some degree of hearing loss. [38] permanent ototoxicity was seen in more than 50% of patients who received cisplatin in doses of greater than 400mg/m2 cumulative dose . Pediatric patients were studied for cisplatin ototoxicity by use of otoacoustic emissions 19 The antioxidant enzymes have been shown to be decreased in association with cisplatin-induced ototoxicity in the rat and Increased hydrogen peroxide formation has been detected in the inner ear after cisplatin exposure. The primary target of cisplatin ototoxicity seems to be the outer hair cells, with the hair cells in the basal turn being most susceptible. This increased susceptibility may result from the relatively low stores of glutathione in the outer hair cells of the basal turn compared with the inner hair cells and the outer hair cells in the more apical turns. 20 One concern about the use of systemic protective agents, such as antioxidants, is the possible intratympanic administration of the protective agent,like dexamethason ,in annimal study was effectiv. 21 A clinical study reported that, contrary to previous studies in the literature, amifostine (600 mg/m2 ), given as an intravenous bolus immediately before and 3 hours after cisplatin and craniospinal irradiation in children with medulloblastoma,provided significant protection against hearing loss. 22 Carboplatin is a newer platinum compound that has been found to be less nephrotoxic than cisplatin. Carboplatin may be more ototoxi than initial studies indicated. High-dose carboplatin (2 g/m 2 total dose) was associated with hearing loss in 9 of 11 children (82%), with hearing losses in the speech frequencies that were sufficiently severe that hearing aids were recommended. 23 DFMO was developed as an irreversible inhibitor of the enzyme ornithinedecarboxylase. It was hoped to be effective in chemotherapy for hyperproliferative diseases, including cancer and certain infectious processes. DFMO was found to cause treatment-limiting, but reversible ototoxicity at high doses. 24 The most commonly used Furosemide Ethacrynic Acid ,Torsemide Bumetanide, Seem to have ototoxic effect on Stria Vascularis with little hair cell damage. Synergistic effect with aminoglycosides. Clinical studies suggested that the ototoxicity of furosemide may be reduced by infusing the drug at rates of less than 15 mg/min. Bumetanide and Torsemide are new loop diuretices, were found to cause reversible hearing loss in cats at a dose that was similar to that of furosemide No evidence of ototoxicity has been shown in humans to date. 25 Hydrocodone is a narcotic analgesic. It is often combined with acetaminophen, and is frequently prescribed Common adverse reactions to this combination analgesic drug include dizziness, nausea, vomiting, drowsiness, and euphoria 26 Hydrocodone abuse was associated with rapidly progressive SNHL in 12 patients reported from the House Ear Clinic. In four patients, the initial presentation was unilateral, and two patients also experienced vestibular symptoms. 27 1. The clinician should document rapidly progressive SNHL that is bilateral. 2. There should be no vestibular symptoms (although this is contradicted in a previous series). 3. There should be no response to steroid therapy. Ototoxicity increase by genetic predispose and hepatitis c 28 Toxicity likely due to decreased perfusions of the inner ear structures. Ototoxic Effects Tinnitus Hearing Loss. Vertigo Nausea Usually SNHL, with 4kHz notch,and Usually reversible upon cessation, but can be permanent. 29 Salicylic acid enters the cochlea causing perilymph levels to equal serum levels. Toxicity appears to be related to metabolic, not morphologic, changes in cochlea. Side Effects: . Tinnitus . Hearing Loss . Flat, Symmetric mild to moderate SNHL .. Recovery generally occurs 24‐72 hours after stopping drug 30 Hearing loss in humans may be related to the concentration of salicylates in blood. Patients with blood levels Tinnitus seems to increase continuously with increasing plasma concentrations of salicylate over 40 to 320 mg/dL 31 Most cases of hearing loss and tinnitus have been transient recovery of normal hearing usually occurs within 1 to 2 weeks after stopping erythromycin. Two cases of permanent ototoxicity have been reported, however: one with permanent tinnitus and one with permanent hearing loss. Hearing loss from erythromycin has been reported in liver or kidney transplant patients. The incidence of hearing loss in these patients seems to be dose related 32 Three patients complained of hearing loss, and audiograms confirmed mild to moderate SNHL, which resolved within 2 to 4 weeks after cessation of treatment. [162] Another series of patients had reversible ototoxicity associated with high-dose oral azithromycin therapy (2400 mg/day). It took an average of 5 weeks for hearing to recover after cessation of treatment. 33 low-dosage or short-duration treatment protocol for aminoglycosides with no clinical risk factors, the use of a pretreatment and posttreatment audiogram with a weekly selfassessment checklist monitoring may be adequate, but would not provide an early warning of potential hearing loss. [183] 34 For cisplatin treatment protocols, monitoring at baseline, just before beginning each cycle, when the patient is less ill and is able to cooperate better, and after the conclusion of therapy may be sufficient to document hearing loss and provide guidelines for rehabilitation because lifesaving therapy may impossible to modify As protective agents progress into clinical use, high-frequency audiometry may help to monitor the efficacy of the protective protocols. 35 For a high-risk, long-duration course of therapy with a high-risk agent such as amikacin, pretreatment and post-treatment testing with intervening weekly or biweekly monitoring of conventional audiometry and high-frequency audiometry may be advisable. For aminoglycosides, the final audiogram after treatment should not be performed until a few weeks after conclusion of therapy because additional delayed effects on hearing may occur. 36 (1) Pretreatment audiograms should be obtained in elderly patients and in patients who have impaired liver or kidney function. (2) Caution should be used in combining erythromycin with other potentially ototoxic drugs. (3) If the serum creatinine is greater than 180 mol/L, the daily dose of erythromycin should not be greater than 1.5 g. 37 Thanks for your attention!