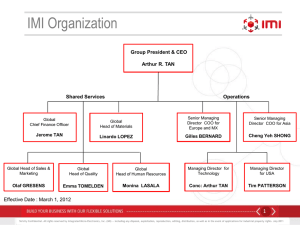
Translational Approaches To T1DM
advertisement

From IMI to IMI2 Hugh Laverty Senior Scientific Project Manager Vilnius, 11th September 2014 The way in which pharmaceutical companies develop new medicines is changing Regulators Rising R&D cost EU Pricing Pharma Patent cliff Generics Declining R&D productivity HC Reform New approaches needed “Deciphering the complexity of human diseases and finding safe, cost-effective solutions that help people live healthier lives requires collaboration across scientific and medical communities throughout the health care ecosystem. Indeed, we must acknowledge that no single institution, company, university, country, or government has a monopoly on innovation.” Innovative Medicines Initiative: Joining forces in the healthcare sector The biggest public/private partnership in Life Science aiming to: • Make drug R&D processes in Europe more innovative and efficient • • Enhance Europe’s competitiveness Address key societal challenges Features: • • 1:1 funding, joint decision making • Large pharmaceutical industry, represented by EFPIA, contributes in-kind All EU funds go to SMEs, academia, patient organisations and regulatory agencies How it works SMEs Academic research teams Step 1 Topic Definition & Launch Hospitals Step 2 EFPIA EoI Submission Step 3 Applicant Consortia IMI Regulatory authorities 18 weeks Step 5 Governing Board approval IMI Signatures & project kick-off Patients’ organisations GB approval of 1st ranked FPP negotiations start 1st ranked EoI Selection Call Launch Full Project Proposal Submission Step 4 1st ranked EoI + EFPA 9 weeks Signatures & project kick-off 6 weeks The Consortium The IMI community 46 projects Calls 1-8 > 6000 researchers 410 EFPIA teams 714 academic & research teams 61% of projects reported some form of PATIENT INVOLVEMENT 23 patient org. 135 SMEs 14 regulators REGULATORS ON BOARD OF 50% of projects have 12 REGULATORY AUTHORITIES PROJECTS representatives in Scientific Advisory Boards The IMI portfolio Calls 1-8 participations per country IMI projects IMI scientific output 708 3709 2.04 19% PUBLICATIONS CITATIONS CITATION IMPACT HIGHLY CITED Number of publications per year 350 300 250 200 150 100 50 0 2009 2010 2011 2012 2013 Making a difference Implementation of project results inside industry Project IMIDIA Results description Area diabetes The human beta cell line EndoC BetaH1 has been validated by Endocells and 3 pharma partners confirming their initial insulin secretion capacity. These cells have been successfully transferred as a research tool for drug discovery to industrial partners. DDMORE knowledge management Several drug/disease models identified by DDMORE are adopted or eTRIKS knowledge management Adoption of the eTRIKS results, TransMART system deployments in 5 further developed inside the industry. pharmaceutical companies. Preclinical model of spontaneous pain in rodents has been developed, standardized, validated, and is already used for internal decision EUROPAIN Chronic pain making in the drug development process. The ultraviolet B (UVB) pain model has also started to be used for in house R&D. Impact on regulatory framework Project Area PROactive COPD EU-AIMS autism Results description Qualification Advice completed at the EMA Started EMA formal scientific advice procedure for qualification of 5 biomarkers in ASD Provided an update on the eTOX database and the prediction system to the CHMP eTOX drug safety Safety Working Party (SWP) at EMA. Scientific Advice Procedure was initiated. MARCAR cancer Has developed new biomarkers, technologies, and alternative test systems that help explain or predict animal and/or human carcinogenic pathways and mechanisms for non-genotoxic carcinogenesis. This will provide enhanced scientific rationale for Carcinogenicity Assessment Document (CAD) submissions, with potential impact for ICH S1 carcinogenicity testing guideline revisions. Safe-T DDMORE drug safety Developed and now progressed towards an aligned EMA/FDA qualification a set of novel safety biomarkers for drug-induced kidney, liver, and vascular injury. knowledge management In May 2012 an advisory meeting with EMA and FDA representatives was held. Through a Modelling Review Group , DDMoRe is in regular contact with both the EMA and FDA regarding the qualification of the content of the project’s Model Library. SME participation in IMI projects (up to 8th Call) Total IMI commitment € 723 million Total received by SMEs € 133 million % SME 18.4% Total IMI participations 886 Total SME participations 135 % SME 15% SME success stories SME involved in SAFE-T project “Thanks to IMI our company went from 6 to 50 employees. Now we are ready to go to further expand.” SME involved in IMIDIA project – “1st product released to the market in 2013 – IMI was instrumental in validation of the first cell line product, 2nd product release planned this year, 3rd diagnostic product in development. In preparation: a new patent filing to protect technologies for the creation of third generation human beta cell lines. SME involved in PharmaCog project “We are developing a blood panel for AD for diagnosis, stratification and companion diagnostics in AD. The Panel was tested on 300 patients in IMI project” SME involved in eTOX project “We have developed in silico models for predicting toxicity, which were validated by pharmas in eTOX. Now we have signed a contract with one of the companies to use our models in house.” Promoting patient involvement IMI makes efforts to enhance patient centric approach − Patient dedicated workshops − Involving patients at all levels − Providing forum for discussion IMI best practice examples: EUPATI U-BIOPRED PROactive For patient-centric R&D more trained patients are needed Competent authorities Public Trial protocol design, informed consent, ethical review, marketing authorization, value assessment, health policy Policy makers Driving force Co-researcher Reviewer Advisor Info provider Research subject Research Ethics Committees HTA agencies & committees Clinical Research Paradigm shift in empowering patients on medicines R&D Key European initiative to provide objective, credible, correct and up-to-date public knowledge about medical research Will build competencies & expert capacity among patients & public Will facilitate patient involvement in R&D to collaborate in academic research, industry research, authorities and ethics committees Collaboration Key collaborative activity areas: Diabetes, CNS disorders, Tuberculosis, Patient Reported Outcomes, Cancer, Preclinical Safety and Education & Training. IMI signed horizontal agreements with: Critical Path, Juvenile Diabetes Research Foundation as well as with Clinical Data Interchange Standards Consortium. The measures of success SUCCESS New models developed & published Setting new standards In house implementation by industry Better Science = Better Decisions Impact on regulatory guidelines IMI’s drug discovery platforms European Lead Factory Focus: identification of new hits ELF Budget: €92.0m EFPIA in-kind €80.0m IMI JU ‘Qualified’ hit European Lead Factory Target screening www.europeanleadfactory.eu Hit-to-lead Lead-tocandidate Preclinical Phase I Phase II Phase III ND4BB Drug Discovery Platform Lead nd4bb-enable.eu Clinical Candidate ENABLE Focus: to move promising hits into early clinical development Phase 1ready ENABLE Budget: €26.0m EFPIA in-kind €58.9m IMI JU Towards IMI2 The Evolution of IMI: From bottlenecks in industry – to bottlenecks in Industry and Society Make Drug R&D processes in Europe more efficient and effective and enhance Europe’s competitiveness in the Pharma sector Basic research Idea generation and non-clinical testing Primary focus of early IMI calls 2007 SRA Regulatory Human testing Approval HTA and Pharmacovigilance Shift to also addressing challenges in in society and healthcare 2011 SRA Daily Medical practice IMI 2 includes real life medical practice 2013 SRA The Vision for IMI2 – The right prevention and treatment for the right patient at the right time effective A effective Dx Test not effective Biologically heterogeneous patient population Adverse events Trial and Error e.g. biomarker vs not effective adverse events B C Information based treatment decisions Graphic adapted from C. Carini, C. Fratazzi, Eur. Pharm. Rev. 2008, 2, 39-45 Science is driving advances in diagnosis: breast cancer is actually 10 different diseases Thursday April 19 2012 “A landmark study has reclassified the country’s most common cancer in breakthrough research that could revolutionise the way we treat breast tumours… scientists found breast cancer could be classified into 10 different broad types according to their common genetic features.” http://www.nhs.uk/news/2012/04april/Pages/breast-cancer-genetic-diversity-mapped.aspx 24 Objectives of IMI2 – what the Regulation says • increase the success rate in clinical trials • where possible, reduce the time to reach clinical proof of concept in medicine development • develop new therapies for diseases for which there is a high unmet need and limited market incentives • develop diagnostic and treatment biomarkers for diseases clearly linked to clinical relevance and approved by regulators; • reduce the failure rate of vaccine candidates in phase III clinical trials through new biomarkers for initial efficacy and safety checks; • provide support for the development of tools, standards and approaches to assess efficacy, safety and quality of regulated health products. The premises • Alignment with Horizon 2020 objectives of the Health challenge • Addressing healthcare priorities identified by the WHO 2013 report on priority medicines for Europe and the world • Strategic Research Agenda aimed at progressing the vision of personalised medicines, for both prevention and treatment • Collaboration across sectors to harness all knowledge and technologies which can contribute to IMI2 vision - diagnostics, imaging, IT, medical devices, … 1.3. Building on the strengths of Europe...... 1.4. Learning from the Innovative Medicines 2.Establishing the Research Priorities for IMI2 ........ 2.1. Challenges facing the healthcare ecosys 2.2. The role of Research & Development in 2.3. Regulatory, health technology assessme 3.Research Objectives of IMI2 ................................ 3.1. Four major axes of research .................. 3.1.1. Axis 1: Target validation and biomarker 3.1.2. Axis 2: Adoption of innovative clinical tr 3.1.3. Axis 3: Innovative Medicines ................. 3.1.4. Axis 4 : Patient tailored adherence prog 4.Enabling Technologies ........................................ 4.1. Excellence in Data Management ........... 5.Implementation strategies .................................. 5.1. Education andinTraining required to imp Therapeutic Areas IMI2 SRA Excellence in clinical trial implementatio (no5.2. priority order) 6.European Health Priorities to be addressed by IM Europe Antimicrobial resistance ........................ World 6.1. 6.2. Osteoarthritis ........................................ 6.3. Cardiovascular diseases ......................... 6.4. Diabetes ................................................. 6.5. Neurodegenerative diseases ................. 6.6. Psychiatric diseases ............................... 6.7. Respiratory diseases .............................. 6.8. Autoimmune diseases ........................... 6.9. Ageing-associated diseases ................... 6.10. Oncology ................................................ 6.11. Rare/Orphan Diseases ........................... 6.12. Vaccines ................................................. 7.Translating research to tangible benefits for Euro 2013: WHO report on priority medicines for Europe and the World: societal challenge reflected in the IMI2 SRA WHO report: Percentage of DALYs for top 20 high burden diseases and conditions 12 10 8 6 4 Tuberculosis Malaria Birth asphyxia and birth trauma Prematurity and low birth weight Breast cancer Colon and rectum cancer Cirrhosis of the liver Osteoarthritis Trachea, bronchus, lung cancer Alzheimer and other dementias Lower respiratory infections Alcohol use disorders HIV/AIDS COPD Hearing loss Diabetes mellitus Unipolar depression Cerebrovascular Ischaemic heart disease 0 Neonatal infections and other… 2 IMI2: Major Axes of Research Biomarker identification/validation (precision medicine) Innovative methodologies to evaluate treatment effect Reclassification of disease by molecular means Target Identification and validation(human biology) Target & Biomarker Identification (safety & efficacy) Determinants of drug /vaccine Safety and efficacy Innovative drug delivery methodologies Innovative clinical trial paradigms European Health Priorities Innovative Medicines Manufacturing for personalised medicines Discovery and Development of novel preventative and therapeutic agents Adoption of innovative clinical trial designs Benefit/Risk Assessment Patient tailored adherence programmes Healthcare delivery: focus on the treatment programmes not just the medicine Innovative adherence programmes Drive change in delivery of medical practice Strategic Research Agenda Comprehensive framework for a 10-year programme Prepared with input from 80+ organisations (internet and targeted) Project ideas from industry and third parties will be screened against it http://goo.gl/jqMP9g IMI2 - Broad participation to be able to set ambitious goals IMI is evolving, with a stronger focus on the needs of patients and society and with simpler rules and procedures Evolution in scientific focus • Stronger focus on needs of patients and society, including unmet needs • Increased emphasis on improving patient access to innovative medicines (in addition to medicines development) • Focus on personalised medicine (the right treatment for the right patient at the right time) IMI2 - Broad Participation to achieve ambitious goals: Bigger budget: 3,45 Billion Euro, equally shared by EU and industry Not limited to EFPIA members: open for other industries / companies, which can contribute to the PPP goals (Healthcare IT, medical devices,…) giving them the opportunity to establish their own projects The principle of large companies providing an inkind contribution matched by IMI funding for public beneficiaries will be retained. IMI2 - Broad Participation to achieve ambitious goals: Specified Budget: 225 million Euros reserved for non-EFPIA led projects (to be matched by inkind contributions) • Objectives, deliverables and timelines determined by the company(ies) proposing the project • Inkind contribution determined by the company(ies) • Once approved by IMI’s Governing Board the Programme Office will launch a call for proposals to identify public partners for the project • The call process and review of submitted proposals will be independent of the company(ies) The Role Of The Programme Office A neutral broker: To implement programmes and activities in the common interest of all stakeholders To monitor the use of public funds and industry investment To guarantee fair and reasonable conditions for optimal knowledge exploitation and dissemination To facilitate the interaction between stakeholders, including Intellectual Property agreements To actively communicate and promote IMI and its activities IMI2: The First Call Two topics: Translational approaches to disease modifying therapy of type 1 diabetes mellitus (T1DM) Magda.Gunn@imi.europa.eu Discovery and validation of novel endpoints in dry agerelated macular degeneration and diabetic retinopathy Nathalie.Seigneuret@imi.europa.eu Submission date: 12 November 2014 Translational approaches to disease modifying therapy of type 1 diabetes mellitus (T1DM) Vilnius, 11th September 2014 Translational Approaches To T1DM: Background • A chronic disease affecting worldwide around 17 Million people and with highest incidence rate in Europe ( ~ 22 / 100.000/ year), with major regional differences. • The incidence of childhood T1DM is reported to be rising rapidly worldwide, especially in the under 5 year old age group. • T1DM is generally seen today as an autoimmune disease, but its cause is unknown (genetic susceptibility, diabetogenic trigger(s) and/or exposure to a driving antigen). • The disease is currently not preventable and no cure is available. The only available pharmacotherapy for T1DM patients is the lifelong injection of insulin. Translational Approaches To T1DM: Aims and Objectives Better Disease Biology and Translational Medicine (Target & Biomarker Identification) • Generation of a high quality and comprehensive European network of clinical and translational research centres (providing a prospective clinical trials database for T1DM) including at risk and early T1DM patients. • Establishment of systematic large-data repository enabling extensive cross functional data mining and integrated data analysis • Phenotypical characterization (in silico based on medical records as well as active through experimental medical studies) • Systematic prospective and retrospective launch of broad “–omics” characterization of human biological samples • Development and characterization of the most appropriate preclinical T1DM model(s) for discovery of novel clinical therapies. Translational Approaches To T1DM: Aims & Objectives Innovative clinical trial paradigms for preventative and disease modification trials in T1DM. • Development of standardized entry criteria and endpoints for T1DM trials (both metabolic and immune profiles) with participation of patient advocacy groups, and regulatory authorities. • Implementation of the use of electronic data capture devices to collect an array of “real world data” • Testing and development of novel bio-statistical methodologies applicable to new compositions of relevant end points for T1DM clinical trials. • Evaluation of novel mono- and combination approaches (i.e. combining multiple immune modulatory approaches, immune cell migration modification, immune tolerance inducers, β-cell enhancing therapeutics) in people with T1DM. Translational Approaches To T1DM: Key Deliverables • An improved understanding of the immunological and beta cell biology aspects of T1DM to disentangle its heterogeneity both in at risk and early diagnosed patients and for staging participants in future T1DM clinical trials. • The development of novel and relevant endpoints & readouts for T1DM clinical trial based on clinical & standardised molecular “real world data” obtained from T1DM patients, and on the application of novel bio-statistical methodologies. • Pre-clinical T1DM models with improved translational value. • Improved understanding of the human T1DM disease biology and optimised clinical trial setting to allow testing novel mono- and combination approaches in T1DM. Translational Approaches To T1DM: EFPIA PARTICIPANTS AND ASSOCIATED PARTNERS Sanofi (coordinator), Juvenile Diabetes Research Foundation (JDRF) (co-coordinator), Novo Nordisk, Eli Lilly, GSK, Helmsley Charitable Trust. DURATION OF THE PROJECT The indicative duration of the project is 84 month (7 years). BUDGET EFPIA and associated partners: EUR 17 630 000 IMI2 JU: EUR 17 630 000 Total: EUR 35 260 000 Translational Approaches To T1DM: APPLICANT CONSORTIUM • • • • • • Academic endocrine clinics and associated supporting departments Basic, translational, and clinical researchers from the fields of T1DM autoimmunity and β-cell biology Drug discovery and medical staff in Pharmaceutical Industry and Small and Medium size Enterprises Hands-on data base specialists and big data managers Patient organizations/representatives Experts in regulatory science and health technology assessment preferably representing European health authorities. The project will be expected to establish a T1DM Patient Advisory Committee Translational Approaches To T1DM: Suggested Work Plan • A plan for interactions with Regulatory Agencies/Health Technology Assessment bodies with relevant milestones and appropriate resource allocation should be included • Synergies with other EU and global initiatives, including IMI projects Discovery and validation of novel endpoints in dry age-related macular degeneration and diabetic retinopathy Vilnius 11th September 2014 Novel Endpoints For Retinal Diseases • Retinal diseases among leading causes of blindness worldwide Age-related macular degeneration (AMD): Early form reported to occur in 30% of the population of 75 years and above (over 50% by age 80); late form in 4 8% of the population over 70 years Approximately 93 million affected by diabetic retinopathy (DR) in 2010 • Limited treatment options for dry form of AMD or DR • Major development hurdles: lack of suitable endpoints for early exploratory and pivotal clinical trials, lack of predictive markers and models Novel Endpoints For Retinal Diseases: Aims & Objectives To evaluate novel endpoint candidates for dry AMD and DR: • technical, medical and health economic appropriateness • bridging preclinical and clinical studies. Methods in scope: • • • • • • Visual function testing beyond Best Corrected Visual Acuity (BCVA) Electrophysiology Imaging methods to assess retinal structure Soluble and genetics biomarkers Patient reported outcome tools and Quality of Life-related endpoints A combination of these methods Novel Endpoints For Retinal Diseases: Key Deliverables Generation of robust data resulting from retrospective and/or prospective studies as basis for discussion of regulatory acceptability of the endpoints for future clinical programmes. It is expected that the proposed research program delivers data on: • Technical evaluation of methods (validity, repeatability, reliability, interpretability, translatability and acceptability by patients) • Development of novel methods and tools • Clinical validation of methods/tools in patient studies for dry AMD & DR • Collection of biomarkers for selection of high risk populations • Synergies between dry AMD and DR vs condition-specific aspects Novel Endpoints For Retinal Diseases: EFPIA PHARMA PARTICIPANTS AND OTHER PARTNERS Bayer HealthCare (coordinator), Sanofi, Novo Nordisk, Zeiss DURATION OF THE PROJECT The indicative duration of the project is 60 month (5 years). BUDGET EFPIA and associated partners: EUR 7 000 000 IMI2 JU: EUR 7 000 000 Total: EUR 14 000 000 Novel Endpoints For Retinal Diseases: Setting-up & running of studies required to meet topic’s objectives Multidisciplinary applicant consortium with a track record of • Clinical expertise in ophthalmology • • • • • • • • Clinical research experience Access to patients and databases Public health expertise Health economic expertise Understanding of pre-clinical models in ophthalmology Biomarkers Data management Regulatory, ethics, patients and project management Novel Endpoints For Retinal Diseases: Suggested Work Plan • Architecture for the full proposal to be suggested by the Applicant consortium • Intention to set-up of an Advisory panel to the Consortium comprising payers, regulatory agencies and other relevant expert advisors • Plan for interactions with Regulatory Agencies/Health Technology Assessment bodies expected • Synergies with other EU and global initiatives, including IMI projects IMI2 Info Day Crowne Plaza Hotel, Brussels, Tuesday 30 September 2014 • • • • • Workshops and presentations of topics by the topic writers Overview of IMI 2 funding and intellectual property (IP) rules Tips on applying for funding under IMI 2 Networking opportunities IMI staff on hand to answer questions We warmly encourages small and medium-sized enterprises, mid-cap businesses, patient organisations, regulatory authorities, academic teams, industry, hospitals and other organisations 50 Questions? Hugh.Laverty@imi.europa.eu www.imi.europa.eu