File - Dr Sam`s Rotation
advertisement

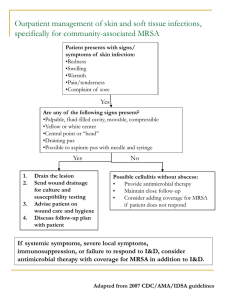
Dalvance (Dalbavancin) A new once-weekly Lipoglycopeptide Antibiotic Prepared by: Kamaldeep Kainth, PharmD 2015 Objectives: ● To define what is MRSA ● To explain current medications for MRSA infection ● To introduce Dalbavancin ● To describe the dosage, storage, Administration, ADRs of Dalbavancin ● To evaluate ABSSSI Trials: Results ● To describe treatment Comparison ● To review prevention of Staph. infection. Patient Case: CA is a 33 y/o male football player admitted to the ER with complaints of fever, inability to walk, Erythema, local swelling and pain on the left leg for about 5 days. His medical history was unremarkable. Physical examination: Wt 180lb, Ht 6ft, T 101.5F, Pain 6/10, local tenderness, restricted leg movement. Labs: SCr 0.9, Glu 101, Hg 12.1, WBC 20, CRP 60. Other labs: WNL Needle Aspiration: GP cocci The patient was initially treated with Cefazolin 3gm/day Patient Case: 2 Days Later... There was no improvement after 2 days of treatment. CA still have fever of 102 and his WBCs are now 25000. Both the needle aspirate and bone culture grew MRSA. How would you treat this patient??? What is MRSA?? MRSA is…. Methicillin Resistant Staphylococcus Aureus. Staphylococcus Aureus, or simply referred as Staph. are bacteria commonly carried on skin or in the nose of healthy person. When staph becomes resistant to Methicillin or Oxacillin antibiotic then its called MRSA... Infections caused by MRSA: ● ● ● ● ● ● Skin and soft tissue infection (most common) Wound (Traumatic) Urinary Tract Infection Osteomyelitis Bacteremia Pneumonia (Less common) Risk Factors: ● ● ● ● ● ● ● Recurrent skin disease or open wound Long term illness or patients on dialysis Illicit drug use (Injectables) Surgery Recent hospitalization. Recent Antibiotic use Living in crowded settings Diagnosis: ...To make a definitive diagnosis and confirm that Staph is the bacteria causing the infection, culture is usually done… ...In the past, most serious Staph bacterial infections were treated with penicillin type antibiotics or a cephalosporins.. What is common among these?? ● ● ● ● ● ● ● ● ● Vancomycin Daptomycin Linezolid Clindamycin Tigecycline Synercid (Quinupristin/Dalfopristin) Telavancin Rifampin Ceftaroline What’s New?? Dalbavancin for injection.. Now Approved for Treatment for Acute bacterial skin and skin structure infections… May 2014…. Introduction: ...Dalbavancin is second-generation semisynthetic lipoglycopeptide antibiotic. ...Because of its longer half-life (t1/2 of 6 10 days) it is dosed once weekly. A complete course of therapy consist of 2 single weekly doses administered on Day 1 and Day 8…. Spectrum of Activity: ● GRAM POSITIVE ORGANISMS: o o o o o o o Staphylococcus Aureus (MSSA and MRSA) Streptococcus pyogenes Streptococcus agalactiae Streptococcus anginosus group (S. anginosus, S. intermedius, S. Constellatus) Enterococcus faecium (Only vanco susceptible strains) Enterococcus faecalis (Only vanco susceptible strains) Also effective against some resistant strains of Streptococcus, Peptostreptococcus species, Clostridium species and Actiniomyces species. Mechanism of Action: Bactericidal ..Dalbavancin interferes with the transpeptidation and transglycosylation step in cell wall synthesis mediated by binding to D-ala D-ala terminus. Binding to this substrate inhibits the cross-linking reactions that strengthen the bacterial cell wall….. Dosage and Administration: ● Acute Bacterial Skin and Skin structure Infection in Adults ● 500mg vial available as sterile powder. ● 1000 mg given IV over 30 minutes on day 1 ● 500 mg given IV over 30 minutes on day 8 ● Must be reconstituted with sterile water for injection and diluted only with 5% dextrose. Renal Impaired: CrCl <30ml/L→ Administer 750 mg on day 1, followed one week later 375 mg Reconstitution and Storage: Each 500 mg vial must be reconstituted with 25 ml of sterile water for injection, USP. The reconstituted solution can then be diluted or transferred to iv bag of 5% Dextrose solution to a final concentration between 1mg/ml to 5mg/ml. Storage: Refrigerated→ 2 - 8 C (36-46 F) Controlled Room temperature→ 20-25 C (6877F) DO NOT FREEZE **Total time from reconstitution to dilution to administration should not exceed 48 hours.** Adverse Effects: Common AEs: ● ● ● ● Constipation (18.2%) Diarrhea (4.4%) Nausea (5.5%) Headache (4.7%) Serious AEs: ● ● ● ● C. Diff Colitis (<2%) GI Hemorrhage (<2%) Infusion reaction (<2%) Hepatotoxicity (<2%) Pregnancy Category: C Drug Interactions: Dalbavancin is not affected by coadministration of known CYP450 substrates, inducers or inhibitors…. Clinical Studies: ABSSSI Trials → 2 Trials performed with total of 1312 randomized patients. The specific infections in these trials were Cellulitis (50%), Major abscess (30%) and wound infections (20%). → Baseline signs and symptoms of infection included: Temperature 38C or higher, WBC 12000 or higher or 10% or more bands. → The mean age was 50 years and mean BMI was 29.1 kg/m2 → Primary endpoint was clinical response rate defined as no increase from baseline in lesion area after 48 to 72 hours and T at or <37.6C Clinical studies: Clinical Response rate in the trials at 48 to 72 hours after initiation of therapy Dalavancin n/N(%) Vancomycin/Lin Difference 95% ezolid n/N (%) CI TRIAL 1 240/288 (83.3%) 233/285 (81.8%) 1.5% (-4.6, 7.9) TRIAL 2 285/371 (76.8%) 288/368 (78.3%) -1.5% (-7.4, 4.6) Dalavance Group: 1000 mg followed 1 week later 500mg. (Dose adjusted for CrCl) Vanco/Linezolid: iv vanco 1000 mg or 15 mg/kg q 12 h. with option to switch to oral linezolid after 3days. Clinical Studies: Secondary end point: → Patients who had reduction in lesions by 20% or greater in 48 to 72 hours after initiation of therapy Dalavance n/N (%) Vanco/Linezolid Difference n/N(%) (95% CI) TRIAL 1 259/288 (89.9%) 259/285 (90.9%) -1.0% (-5.7, 4.0) TRIAL 2 325/371 (87.6%) 316/368 (85.9%) 1.7% (-3.2, 6.7) ADRs: ..In the Clinical trials, the treatment related ADRs for Dalbavancin was 12.3% and for Vanco/Linezolid was 18.3%. **ADRs in > or =3% of patients in Dalbavancin included: Nausea, Diarrhea, Headache, Pruritus. Discontinuations: ● D → 1.8% ● V/L → 2.1% Results: → The study results concluded that Dalbavancin is non-inferior when compared to standard drug therapy (Vancomycin/Linezolid) in Acute Bacterial skin and skin structure infection…. Back to our Patient: CA is a 33 y/o male football player admitted to the ER with complaints of fever, inability to walk, Erythema, local swelling and pain on the left leg for about 5 days. His medical history was unremarkable. There was no improvement after 2 days of treatment with Cefazolin. CA still have fever of 102 and his WBCs are now 25000. Both the needle aspirate and bone culture grew MRSA… What would be the treatment therapy??? Treatment Options: ● ● ● ● ● ● ● ● ● ● Vancomycin <<< Daptomycin Linezolid Clindamycin** Tigecycline Synercid (Quinupristin/Dalfopristin) Telavancin Rifampin Ceftaroline Dalbavancin <<< **CA MRSA only CO$T>>>> ● ● ● ● ● ● ● ● ● ● Vancomycin >>> (750mg/150ml IV $86.98) Daptomycin >>>(500mg IV $403) Linezolid >>> (600mg/300ml IV $167.35) Clindamycin>> (900mg/50ml IV $9.62)*** Tigecycline>> (500mg/100ml IV $1216) Synercid (Quinupristin/Dalfopristin) >>>(350/150mg per vial $307.30) Telavancin >>>>(750mg IV $358.80) Rifampin >>> (600mg IV $136.35) Ceftaroline >>> (600mg IV $758) Dalbavancin<< (750mg vial $1000-$3000)# ***CA-MRSA only Precautions to prevent Staph. Infection... Can I protect myself from becoming infected? ● WASH YOUR HANDS ● If no visible dirt, use alcohol-based hand sanitizer ● Keep cuts and abrasions covered ● Avoid contact with other people’s wounds ● Avoid skin-to-skin contact with infected persons ● Do not share personal items ● Clean objects and other shared surfaces How to keep it from spreading to others? ● Keep infected areas covered ● Follow doctor’s instructions ● Wash hands ● Put disposable waste in a separate trash bag and close tightly before throwing it out ● Wear gloves ● Do not share personal items ● Avoid participating in contact sports Conclusion: Dalbavancin Vancomycin → 2 Dose Regimen for SSTI → Dosed more frequently (qd to q 8 h) → Safety not established in Pediatrics → Established use in neonates, infants and children. → Headache, GI SEs, Hepatic and infusion related SEs (>30min) → Nephrotoxicity, Ototoxicity, Neutropenia, infusion related (>60mins) → Pregnancy Category : C → Pregnancy Category : C → Half-life : 6 to 10 days → Half-life 4 to 6 hours. → Monitoring: Improvement, Diarrhea, Hypersensitivity reaction → Culture, CBS, Improvement, Trough levels, Renal function, Dose adjustments** → Cost: $$$$ → Cost: $$ References: ● http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingM aterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM390793.pdf ● http://www.webmd.com/skin-problems-and-treatments/understandingmrsa-detection-treatment ● http://www.cdc.gov/mrsa/ ● http://www.webmd.com/a-to-z-guides/video/truth-about-mrsa ● http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingM aterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM390793.pdf ● http://www.medscape.org/viewarticle/518862_16 ● http://content.stockpr.com/duratatherapeutics/files/docs/Dalvance+APPRO VED+USPI.PDF ● http://www.duratatherapeutics.com/news-media/pressreleases/detail/333/durata-therapeutics-announces-phase-3-clinical-trial ● http://www-micromedexsolutionscom.proxy.westernu.edu/micromedex2/librarian/PFDefaultActionId/evidenc expert.ShowDrugCompareResults