Landmark Clinical Trials Evaluating NOACS in SPAF - Iqanda-CME
advertisement

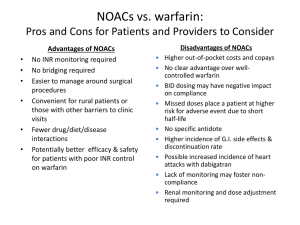
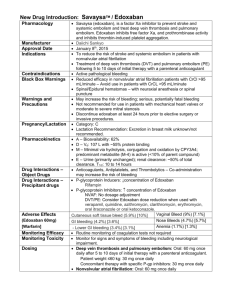
New Paradigms in the Science and Medicine of Heart Disease Deciphering the Maze of Evidence from Landmark Trials Evaluating Non-Monitored, Oral Anticoagulants (NOACs) for SPAF CHRISTIAN T. RUFF, MD, MPH Associate Physician Cardiovascular Division Brigham and Women's Hospital Instructor of Medicine Harvard Medical School Boston, MA History of Warfarin Limitations of Warfarin Delayed onset/offset Multiple food and drug interactions Genetic variability in metabolism (VKORC1 and CYP2C9) Requires frequent monitoring of INR due to limited therapeutic index Preventable Strokes AF Patients with Stroke with no Known Contraindication to Anticoagulation No warfarin 61% INR in range 10% Subtherapeutic INR 29% Gladston, DJ, et al. Stroke 2009;40:235-40 Properties of an Ideal Anticoagulant Properties Benefit Oral, once-daily dosing Ease of administration Rapid onset of action No need for overlapping parenteral anticoagulant Minimal food or drug interactions Simplified dosing Predictable anticoagulant effect No coagulation monitoring Extra renal clearance Safe in patients with renal disease Rapid offset in action Simplifies management in case of bleeding or intervention Antidote For emergencies Comparative PK/PD of NOACs Dabigatran Rivaroxaban Apixaban Edoxaban IIa (thrombin) Xa Xa Xa Hours to Cmax 1-3 2-4 3-4 1-2 Half-life, hours 12-17 5-13 12 10-14 80 33* 27 50 Transporters P-gp P-gp P-gp P-gp CYP Metabolism, % None 32 <32 <4 Target Renal Clearance, % CYP = cytochrome P450; P-gp = P-glycoprotein *33% renally cleared; 33% excreted unchanged in urine Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc. 2013 Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc. 2011 Weinz et al. Drug Dispos Metab 2009;37:1056–1064 ELIQUIS Summary of Product Characteristics. Bristol Myers Squibb/Pfizer EEIG, UK Matsushima et al. Am Assoc Pharm Sci 2011; abstract Ogata, et al. J Clin Pharmacol 2010;50:743–753 Mendell, et al. Am J Cardiovasc Drugs 2013;13:331–342 Bathala, et al. Drug Metab Dispos 2012;40:2250–2255 Ruff CT, et al. Lancet 2013 [in-press] NOAC SPAF Trials Drug # Randomized Dose (mg) Frequency Dose Adjustment At Baseline After Randomization Target INR (Warfarin) Design RE-LY ROCKET-AF ARISTOTLE ENGAGE AF Dabigatran Rivaroxaban Apixaban Edoxaban 18,113 14,266 18,201 21,105 150, 110 20 5 60, 30 Twice Daily Once Daily Twice Daily Once Daily No 20 → 15 5 → 2.5 60 → 30 30 → 15 0 21 5 25 No No No >9% 2.0-3.0 2.0-3.0 2.0-3.0 2.0-3.0 PROBE* 2x blind 2x blind 2x blind *PROBE = prospective, randomized, open-label, blinded end point evaluation Connolly SJ, et al. N Engl J Med 2009;361:1139-1151 Patel MR, et al. N Engl J Med 2011;365:883-891 Granger CB, et al. N Engl J Med 2011;365:981-992 Giugliano RP, et al. N Engl J Med 2013; e-pub ahead of print DOI:10.1056/NEJMoa1310907 Ruff CT, et al. Lancet 2013 [in-press] Pivotal Warfarin-Controlled Trials Stroke Prevention in AF Warfarin vs. Placebo 2,900 Patients NOACs vs. Warfarin 71,683 Patients ROCKET AF (Rivaroxaban) 2010 6 Trial of Warfarin vs. Placebo 1989-1993 RE-LY (Dabigatran) 2009 ENGAGE AF-TIMI 48 (Edoxaban) 2013 ARISTOTLE (Apixaban) 2011 Baseline Characteristics RE-LY (Dabigatran) ROCKET-AF (Rivaroxaban) ARISTOTLE (Apixaban) ENGAGE AF (Edoxaban) # Randomized 18,113 14,264 18,201 21,105 Age, years 72 ± 9 73 [65-78] 70 [63-76] 72 [64-78] Female, % 37 40 35 38 Paroxysmal AF 32 18 15 25 VKA naive 50 38 43 41 Aspirin Use 40 36 31 29 CHADS2 0-1 2 3-6 13 33 32 35 87 30 34 53 47 36 Connolly SJ, et al. N Engl J Med 2009;361:1139-1151 Patel MR, et al. N Engl J Med 2011;365:883-891 Granger CB, et al. N Engl J Med 2011;365:981-992 Giugliano RP, et al. N Engl J Med 2013; e-pub ahead of print DOI:10.1056/NEJMoa1310907 Ruff CT, et al. Lancet 2013 [in-press] Trial Metrics RE-LY (Dabigatran) ROCKET-AF (Rivaroxaban) ARISTOTLE (Apixaban) ENGAGE AF (Edoxaban) Median Follow-Up, years 2.0 1.9 1.8 2.8 Median TTR 66 58 66 68 Lost to Follow-Up, N 20 32 90 1 Metrics *TTR, time in therapeutic range Connolly SJ, et al. N Engl J Med 2009;361:1139-1151 Patel MR, et al. N Engl J Med 2011;365:883-891 Granger CB, et al. N Engl J Med 2011;365:981-992 Giugliano RP, et al. N Engl J Med 2013; e-pub ahead of print DOI:10.1056/NEJMoa1310907 Ruff CT, et al. Lancet 2013 [in-press] RE-LY Efficacy - Dabigatran Stroke/Systemic Embolic Event Non-inferiority Superiority P-value P-value Dabigatran 110 vs Warfarin < 0.001 Dabigatran 150 vs Warfarin < 0.001 0.34 < 0.001 Margin = 1.46 Connolly, et al. N Engl J Med 2009;361:1139-51 0.50 0.75 HR 1.00 1.25 (95% CI) 1.50 RE-LY Efficacy Dabigatran 110 mg Dabigatran 150 mg Stroke/SEE 0.91 (0.74-1.11) 0.66 (0.53-0.82) Ischemic Stroke 1.11 (0.89-1.40) 0.76 (0.60-0.98) Hemorrhagic Stroke 0.31 (0.17-0.56) 0.26 (0.14-0.49) Connolly, et al. N Engl J Med 2009;361:1139-51 0.1 0.3 0.5 Dabigatran Better 1.0 2.0 Warfarin Better RE-LY Safety Results Dabigatran 110 mg Dabigatran 150 mg Major Bleed ICH GI Bleed MI 0.80 (0.69-0.93) 0.93 (0.81-1.07) 0.31 (0.20-0.47) 0.40 (0.27-0.60) 1.10 (0.86-1.41) 1.50 (1.19-1.89) 1.29 (0.96-1.75) 1.27 (0.94-1.71) Connolly, et al. N Engl J Med 2009;361:1139-51 0.1 0.3 0.5 Dabigatran Better 1.0 2.0 Warfarin Better Dabigatran ► 150 mg twice daily if CrCL > 30 mL/min ► 75 mg twice daily if CrCL 15-30 mL/min ROCKET AF Efficacy - Rivaroxaban Stroke/Systemic Embolic Event Rivaroxaban Warfarin On Treatment Event Rate Event Rate HR (95% CI) P-value 1.70 2.15 0.79 (0.65, 0.95) 0.015 2.12 2.42 0.88 (0.74, 1.03) 0.117 N = 14,143 ITT N = 14,171 0.5 1 Rivaroxaban better 2 Warfarin better Event Rates are per 100 patient-years Based on Safety on Treatment or Intention-to-Treat through Site Notification populations Patel, et al. N Engl J Med 2011;365(10);883-891 ROCKET AF Key Secondary Efficacy Rivaroxaban (%/yr) Warfarin (%/yr) Hazard Ratio (95% CI) Pvalue Ischemic Stroke 1.34 1.42 0.94 (0.75-1.17) 0.581 Hemorrhagic Stroke 0.26 0.44 0.59 (0.37-0.93) 0.024 MI 0.91 1.12 0.81 (0.63-1.06) 0.121 Total Mortality 1.87 2.21 0.85 (0.70-1.02) 0.073 Vascular Mortality 1.53 1.71 0.89 (0.73-1.10) 0.289 Event Patel, et al. N Engl J Med 2011; 365(10);883-891 ROCKET AF Safety Rivaroxaban (%/yr) Warfarin (%/yr) Hazard Ratio (95% CI) Pvalue Major and Clinically Relevant Bleed 14.9 14.5 1.03 (0.96-1.11) 0.44 Major Bleed 3.6 3.4 1.04 (0.90-1.20) 0.58 Fatal Bleed 0.2 0.5 0.50 (0.31-0.79) 0.003 ICH 0.5 0.7 0.67 (0.47-0.93) 0.02 Event Patel, et al. N Engl J Med 2011; 365(10);883-891 Rivaroxaban ► 20 mg if CrCl > 50 mL/min ► 15 mg if CrCL 15-50 mL/min ARISTOTLE Efficacy - Apixaban HR 0.79 (0.66–0.95) (1.60 %/yr) 21% RRR (1.27 %/yr ) P (non-inferiority) < 0.001 P (superiority) = 0.011 Granger CB, et al. NEJM 2011; 365:981-992 ARISTOTLE Efficacy Outcomes Apixaban (N = 9120) Outcome Stroke or systemic embolism* Warfarin (N = 9081) Event Event Rate Rate (%/yr) (%/yr) HR (95% CI) P Value 1.27 1.60 0.79 (0.66, 0.95) 0.011 1.19 1.51 0.79 (0.65, 0.95) 0.012 Ischemic or uncertain 0.97 1.05 0.92 (0.74, 1.13) 0.42 Hemorrhagic 0.24 0.47 0.51 (0.35, 0.75) < 0.001 0.09 0.10 0.87 (0.44, 1.75) 0.70 All-cause death* 3.52 3.94 0.89 (0.80, 0.998) 0.047 Stroke, SE, or all-cause death 4.49 5.04 0.89 (0.81, 0.98) 0.019 Myocardial infarction 0.53 0.61 0.88 (0.66, 1.17) 0.37 Stroke Systemic embolism (SE) Granger CB, et al. NEJM 2011; 365:981-992 ARISTOTLE Safety End Points Apixaban (%/yr) Warfarin (%/yr) Hazard Ratio (95% CI) Pvalue ISTH Major Bleeding 2.13 3.09 0.69 (0.60-0.80) < 0.001 ICH 0.33 0.80 0.42 (0.30-0.58) < 0.001 GUSTO Severe 0.52 1.13 0.46 (0.35-0.60) < 0.001 Gastrointestinal 0.76 0.86 0.89 (0.70-1.15) 0.37 Event Granger CB, et al. NEJM 2011; 365:981-992 Apixaban ► 5 mg twice daily ► 2.5 mg twice daily if at least 2 of the following: Age ≥ 80 years Weight ≤ 60 kg Cr ≥ 1.5 mg/dL Primary Endpoint: Stroke / SEE (2.8 years median f/u) Noninferiority Analysis (mITT, On Treatment) Hazard ratio (97.5% CI) Warfarin TTR 68.4% 0.79 Edoxaban 60* mg QD vs warfarin P Values Non-inferiority Superiority P<0.0001 P=0.017 1.07 P=0.005 Edoxaban 30* mg QD vs warfarin 0.50 1.00 1.38 P=0.44 2.0 edoxaban noninferior Superiority Analysis (ITT, Overall) Hazard ratio (97.5% CI) 0.87 Edoxaban 60* mg QD vs warfarin P=0.08 1.13 Edoxaban 30* mg QD vs warfarin P=0.10 0.50 *Dose reduced by 50% in selected pts P Value for Superiority 1.00 2.0 edoxaban superior edoxaban inferior Key Secondary Outcomes Edoxaban 60* mg QD vs warfarin Edoxaban 30* mg QD vs warfarin 0.33 Hem. Stroke Warfarin TTR 68.4% HR (95% CI) P vs warfarin E-60 0.54 E-30 <0.001 <0.001 1.00 1.41 Ischemic Stroke 0.87 0.95 2° EP: Stroke, SEE, CV death Death or ICH 0.92 0.87 CV death 0.86 0.85 Myocardial infarction <0.001 0.005 0.32 0.004 0.87 0.82 All-cause mortality 0.97 <0.001 0.08 0.006 0.013 0.008 0.60 0.94 0.13 1.19 *Dose reduced by 50% in selected pts 0.25 0.5 edoxaban superior 1.00 2.0 edoxaban inferior Main Safety Results - Safety Cohort on Treatment Edoxaban 60* mg QD vs warfarin Edoxaban 30* mg QD vs warfarin Warfarin TTR 68.4% ISTH Major Bleeding Hazard ratio (95% CI) 0.80 0.47 P<0.001 P<0.001 0.55 Fatal Bleeding P=0.006 P<0.001 0.35 0.47 Intracranial Hemorrhage 0.25 *Dose reduced by 50% in selected pts P<0.001 P<0.001 0.30 1.23 Gastrointestinal Bleeding P Value vs warfarin 0.67 0.5 edoxaban superior 1.0 P=0.03 P<0.001 2.0 edoxaban inferior Safety cohort=all patients who received at least 1 dose by treatment actually received All NOACS: Stroke or SEE Risk Ratio (95% CI) 0.66 (0.53 - 0.82) RE-LY [150 mg] ROCKET AF 0.88 (0.75 - 1.03) ARISTOTLE 0.80 (0.67 - 0.95) ENGAGE AF-TIMI 48 0.88 (0.75 - 1.02) [60 mg] Combined 0.81 (0.73 - 0.91) [Random Effects Model] N=58,541 0.5 Heterogeneity p=0.13 p=<0.0001 Favors NOAC 1 Favors Warfarin 2 Ruff CT, et al. Lancet 2013. Published online December 4, 2013 Secondary Efficacy Outcomes Risk Ratio (95% CI) Ischemic Stroke 0.92 (0.83 - 1.02) p=0.10 Hemorrhagic Stroke 0.49 (0.38 - 0.64) p<0.0001 MI 0.97 (0.78 - 1.20) p=0.77 All-Cause Mortality 0.90 (0.85 - 0.95) p=0.0003 0.2 0.5 Favors NOAC 1 2 Favors Warfarin Heterogeneity p=NS for all outcomes Ruff CT, et al. Lancet 2013. Published online December 4, 2013 All NOACS: Major Bleeding Risk Ratio (95% CI) 0.94 (0.82 - 1.07) RE-LY [150 mg] ROCKET AF 1.03 (0.90 - 1.18) ARISTOTLE 0.71 (0.61 - 0.81) ENGAGE AF-TIMI 48 0.80 (0.71 - 0.90) [60 mg] Combined 0.86 (0.73 - 1.00) [Random Effects Model] p=0.06 N=58,498 0.5 Heterogeneity p=0.001 Favors NOAC 1 Favors Warfarin 2 Ruff CT, et al. Lancet 2013. Published online December 4, 2013 Secondary Safety Outcomes Risk Ratio (95% CI) 0.48 (0.39 - 0.59) ICH p<0.0001 1.25 (1.01 - 1.55) GI Bleeding 0.2 p=0.043 0.5 Favors NOAC 1 2 Favors Warfarin Heterogeneity ICH, p=0.22 GI Bleeding, p=0.009 Ruff CT, et al. Lancet 2013. Published online December 4, 2013 Subgroups: Stroke or SEE Age Gender Diabetes Prior Stroke or TIA CrCl CHADS2 Score VKA Status Center-Based TTR Risk Ratio (95% CI) P-Interaction <75 0.85 (0.73 - 0.99) p=0.38 ≥75 0.78 (0.68 - 0.88) Female 0.78 (0.65 - 0.94) Male 0.84 (0.75 - 0.94) No 0.83 (0.74 - 0.93) Yes 0.80 (0.69 - 0.93) No 0.78 (0.66 - 0.91) Yes 0.86 (0.76 - 0.98) <50 0.79 (0.65 - 0.96) 50-80 0.75 (0.66 - 0.85) >80 0.98 (0.79 - 1.22) 0-1 0.75 (0.54 - 1.04) 2 0.86 (0.70 - 1.05) 3-6 0.80 (0.72 - 0.89) Naive 0.75 (0.66 - 0.86) Experienced 0.85 (0.70 - 1.03) <66% 0.77 (0.65 - 0.92) ≥66% 0.82 (0.71 - 0.95) 0.5 1 Favors NOAC p=0.52 p=0.73 p=0.30 p=0.12 p=0.76 p=0.31 p=0.60 2 Favors Warfarin Ruff CT, et al. Lancet 2013. Published online December 4, 2013 Subgroups: Major Bleeding Age Gender Diabetes Prior Stroke or TIA CrCl CHADS2 Score VKA Status Center-Based TTR 0.2 Risk Ratio (95% CI) P-Interaction <75 0.79 (0.67 - 0.94) p=0.28 ≥75 0.93 (0.74 - 1.17) Female 0.75 (0.58 - 0.97) Male 0.90 (0.72 - 1.12) No 0.71 (0.54 – 0.93) Yes 0.90 (0.78 - 1.04) No 0.85 (0.72 - 1.01) Yes 0.89 (0.77 - 1.02) <50 0.74 (0.52 - 1.05) 50-80 0.91 (0.76 - 1.08) >80 0.85 (0.66 - 1.10) 0-1 0.60 (0.45 - 0.80) 2 0.88 (0.65 - 1.20) 3-6 0.86 (0.71 - 1.04) Naive 0.84 (0.76 - 0.93) Experienced 0.87 (0.70 - 1.08) <66% 0.69 (0.59 - 0.81) ≥66% 0.93 (0.76 - 1.13) 0.5 Favors NOAC 1 p=0.29 p=0.12 p=0.70 p=0.57 p=0.09 p=0.78 p=0.022 2 Favors Warfarin Ruff CT, et al. Lancet 2013. Published online December 4, 2013 ACTIVE-W: Stroke or SEE TTR ≥ 65% TTR < 65% 0.10 RR = 1.83 RR = 1.11 P = 0.47 0.08 P < 0.0001 0.06 0.04 Clopi + ASA C+A OAC VKA 0.02 0.02 0.04 0.06 Clopi + ASA C+A OAC 0.0 VKA 0.0 Event Rate (%) 0.08 0.10 P-interaction = 0.013 0.0 0.5 1.0 Years Connolly SJ, et al. Circulation 2008;118:2029-2037 1.5 0.0 0.5 1.0 Years 1.5 ACTIVE-W: Major Bleeding TTR ≥ 65% TTR < 65% RR = 0.68 P = 0.08 0.03 0.03 0.04 P = 0.027 OAC OAC C+A 0.02 0.02 C+A 0.01 C+A C+A 0.0 0.01 OACOAC 0.0 Event Rate (%) 0.05 RR = 1.55 0.04 0.05 P-interaction = 0.0006 0.0 0.5 1.0 Years Connolly SJ, et al. Circulation 2008;118:2029-2037 1.5 0.0 0.5 1.0 Years 1.5 Low Dose Regimens Efficacy & Safety Outcomes Dabigatran 110 mg & Edoxaban 30 mg Risk Ratio (95% CI) 1.03 (0.84 - 1.27) Stroke or SEE p=0.74 1.28 (1.02 - 1.60) Ischemic Stroke p=0.045 0.33 (0.23 - 0.46) Hemorrhagic Stroke p<0.0001 MI 1.25 (1.04 - 1.50) p=0.019 0.89 (0.83 - 0.96) All-Cause Mortality p=0.003 0.65 (0.43 - 1.00) Major Bleeding p=0.05 0.31 (0.24 - 0.41) ICH p<0.0001 0.89 (0.57 - 1.37) GI Bleeding p=0.58 N=26,107 Heterogeneity P=NS for outcomes except: Major Bleeding, p=<0.001 GI Bleeding, p=0.01 0.2 0.5 Favors Low Dose NOAC 1 2 Favors Warfarin Ruff CT, et al. Lancet 2013. Published online December 4, 2013 Cardioversion RE-LY % (N=647) (N=672) Nagarakanti R, et al. Circulation 2011;123:131-136 (N=664) Cardioversion & Catheter Ablation ROCKET AF % Piccini JP, et al. JACC 2013;61:1998-2006 % Catheter Ablation with NOACs Case-control: 763 consecutive patients undergoing AF ablation P=0.85 P=0.81 P=1.0 P=1.0 Kim JS, et al. Heart Rhythm 2013; 10:483-489 Periprocedural Major Bleeding RE-LY % Healey JS, et al. Circulation 2012; 126:343-348 Discontinuing NOACs Prior to Procedures Dabigatran Apixaban Edoxaban Rivaroxaban No important bleeding risk and/or adequate local haemostasis possible: perform at trough level (i.e. ≥12h or 24h after last intake) Low risk High risk Low risk High risk Low risk High risk Low risk High risk CrCl ≥80 ml/min ≥24h ≥48h ≥24h ≥48h no data no data ≥24h ≥48h CrCl 50-80 ml/min ≥36h ≥72h ≥24h ≥48h no data no data ≥24h ≥48h CrCl 30-50 ml/min ≥48h ≥96h ≥24h ≥48h no data no data ≥24h ≥48h ≥36h ≥48h no data no data ≥36h ≥48h not not CrCl 15-30 indicated indicated ml/min CrCl <15 ml/min no official indication for use www.NOACfor AF.eu Heidbuchel H, et al. Eurospace 2013;15:625-651. Dabigatran (FIIa) Monitoring aPTT Van Ryn J, et al. Thromb Hemost. 2010; 103:1116-1127 Hemoclot Test Rivaroxaban (Fxa) Monitoring PT Rotachrom Anti-Xa Prothrombin time (s) 40 30 20 10 0 0 100 200 300 400 Rivaroxaban (µg L-1) 500 600 Van Ryn J, et al. Thromb Hemost. 2010; 103:1116-1127 Managing Bleeding with NOACs www.NOACfor AF.eu Heidbuchel H, et al. Eurospace 2013;15:625-651. ARISTOTLE Apixaban: Increased Events at End of Trial Stroke or systemic embolism (n) 25 20 Apixaban to VKA Group Warfarin to VKA Group Apixaban to VKA Total Events Warfarin to VKA Total Events 21 15 10 5 5 0 1-2 3-7 8-14 15-30 Days after last dose Pattern mirrored the first 30 days of the trial where warfarin-naïve patients starting warfarin had a higher rate of stroke or systemic embolism (5.41%/year) than warfarin-experienced patients (1.41%/year). Granger CB, et al. European Heart Journal 2012;33 (Supplement):685-686 ROCKET AF Rivaroxaban: Increased Events at End of Trial 81.3 # Primary Events Warfarin P= 0.008 48.8 Rivaroxaban Rivaroxaban Warfarin # Primary Events during first 30 days of transition Patel MR, et al. NEJM 2011; 365:883-891 Patel MR, et al. JACC 2013;61:651-658 Safety/Days 3 to 30 after the last dose Key Transition Plan Components Double-Blind Phase Transition Period Duration of Anticoagulant Effect Warfarin Long NOAC INCREASED RISK OF STROKE INCREASED RISK OF STROKE Short Solution = Transition Plan Components: 1. Open-Label OAC 2. Transition Kit 3. Frequent INRs 4. VKA Algorithm All Patients: Stroke or SEE 30 Day Transition Period Warfarin # Patients with Stroke or SEE 8 Edoxaban High-Dose 7 Edoxaban Low-Dose 6 5 4 3 Warfarin Edoxaban HD Edoxaban LD 7 Strokes (6 Isch, 1 Hem) 7 Strokes (6 Isch, 1 Hem) 7 Strokes (6 Isch, 1 Hem) 2 1 0 Days after End of Trial Visit HR 1.00 p=0.99 HR 0.98 p=0.96 All Patients: Major Bleeding 30 Day Transition Period Warfarin # Patients with Major Bleed 20 Edoxaban High-Dose 18 Edoxaban Low-Dose 16 14 12 10 8 6 4 2 Warfarin 11 Major Bleeds Edoxaban HD 10 Major Bleeds Warfarin Bleeds Edoxaban LD 6 Major 18 Major Bleeds Edoxaban HD 4 Major Bleeds Edoxaban LD 5 Major Bleeds HR 0.90 p=0.82 HR 1.60 p=0.22 Transition Kit 0 Days after End of Trial Visit RELY-ABLE Stroke or SEE Dabigatran 150 mg: 1.46 % / year Dabigatran 110 mg: 1.60 % / year HR 0.91 (0.69-1.20) Connolly SJ, et al. Circulation 2013;128:237-243 Major Bleeding Dabigatran 150 mg: 3.74% / year Dabigatran 110 mg: 2.99% / year HR 1.26 (1.04-1.53) Postmarketing Reports of Bleeding with Dabigatran Southworth MR, et al. N Engl J Med 2013; 368(14):1272-1274 Outcomes of Major Bleeding with Dabigatran or Warfarin 5 phase III AF and VTE trials: 1,034 individuals Adjusted OR 0.66 (95% CI 0.46-1.00) 13.0% p=0.05 9.1% Patients with bleed on dabigatran: RBC transfusion Plasma & Shorter ICU Stay Majeed A, et al. Circulation 2013 [published online September 30, 2013] Conclusions New therapies provide the promise of providing safer and more convenient anticoagulation. There are important differences in the half-life, metabolism, & renal elimination across the NOACs that will alter the risk/benefit profile in specific populations. Persistent concern with lack of ability to monitor the level of anticoagulation. Further experience and guidance needed in managing anticoagulation peri-procedure. Desire for reversal agent and strategies to manage serious bleeding.