Atrial Fibrillation & Anticoagulants
advertisement
Atrial Fibrillation &
Anticoagulants
Margaret Jin, BScPhm, PharmD, MSc, CDE
Hamilton Family Health Team
May 27, 2014
Disclosure
I have no actual or potential conflict of
interest in relation to this presentation
Outline
Case Presentation
Canadian Cardiovascular Society 2012
Recommendations
Dabigatran (Pradaxa®)
Rivaroxaban (Xarelto®)
Apixaban (Eliquis®)
Summary
Questions
Case
Mr. AF, a 70 y male with Hypertension (BP=135/85)
and history of GERD. He was just diagnosed with
non-valvular permanent atrial fibrillation
Normal renal and liver function
Current meds:
◦
◦
◦
◦
◦
Ramipril 10mg once daily
Bisoprolol 5mg once daily
Amlodipine 5mg once daily
Rabeprazole 20mg once daily
No OTCs
Smokes 25 cigs/d x 55 years, drinks no alcohol
ODB drug plan
BP=Blood Pressure, GERD=GastroEsophageal Reflux Disease, ODB=Ontario
Drug Benefit, OTCs=Over-the-counters
Anticoagulation options
What anticoagulant (if any), would you
give?
◦
◦
◦
◦
◦
◦
None?
Aspirin?
Warfarin?
Dabigatran?
Rivaroxaban?
Apixaban?
Assess Thromboembolic Therapy
Three Steps
1. Assess Thromboembolic Risk
a. CHADS2 Risk Criteria
2.
Assess Bleeding Risk
a. HAS-BLED Risk Criteria
3.
Assess Benefit vs. Risk
1. Assessing Thromboembolic Risk
CHADS2 Risk Criteria
Points
Congestive Heart Failure
1
(symptoms in the last 3 months)
Hypertension (diagnosis)
1
Age ≥ 75 years
1
Diabetes mellitus
1
Stroke/Transient Ischemic Attack (prior)
2
What is Mr. AF’s CHADS2 score?
Recommended Therapy
CHADS2
Stroke
Rate %/yr
Canadian Cardiology Society (CCS) 2012
Recommendations
0
1.9
No additional risk factors: No antithrombotic
Female or vascular disease: ASA 75-325mg daily
Age ≥ 65 yrs or female & vascular disease: OAC
1
2.8
OAC preferred
Alternatives: ASA 75-325mg daily
2
4
3
5.9
4
8.5
5
12.5
6
18.2
Oral anticoagulant (OAC)
When OAC is indicated, most patients
should receive dabigatran, rivaroxaban, or
apixaban in preference to warfarinCCS 2012
ASA=Acetylsalicylic Acid, OAC=oral anticoagulant
2. Assessing Bleeding Risk
HAS-BLED Risk Criteria
Hypertension (SBP > 160 mmHg)
Abnormal renal (transplantation, dialysis, SCr > 200umol/L) or
liver function (AST/ALT>3xULN, bilirubin>2xULN) (1 point each)
Points
1
1 to 2
Stroke (caused by a bleed)
1
Bleeding (hospitalization, decrease Hgb > 20g/L, transfusion)
1
Labile INRs (therapeutic range < 60%)
1
Elderly (age > 65 years)
1
Drugs (ASA/NSAID) or alcohol (≥8 drinks/week) (1 point each)
1 to 2
What is Mr. AF’s HAS-BLED score?
ASA=acetylsalicylic acid, AST=aspartate aminotransferase, ALT=alanine aminotransferase,
Hgb=hemoglobin, INRs=international normalized ratios, NSAIDS=non-steroidal anti-inflammatory drugs,
SCr=serum creatinine, ULN=upper limit of normal
HAS-BLED Score & Major Bleeds
HAS-BLED
Score
Major Bleeds
(%/yr)
0
1
2
1.13
1.02
1.88
3
4
5
3.74
8.70
12.50
Major bleed
Intracranial,
hospitalization,
decrease Hgb >
20g/L, +/transfusion
NOTE:
HAS-BLED Score & Major Bleed risk is only validated with warfarin
3. Assess Risk vs. Benefit – Mr. AF
CHADS2 = 1 = 2.8%/yr Stroke rate
HAS-BLED = 1 = 1.02%/yr Major bleed
Risk of stroke > Major Bleed Risk
Recommendation: Oral anticoagulants
◦
◦
◦
◦
Warfarin
Dabigatran
Apixaban
Rivaroxaban
Preferred by Canadian Cardiology
Society 2012 guidelines
ODB – Limited Use for newer agents
ODB=Ontario Drug Benefit
Ontario Drug Benefit – Limited Use
For the prevention of stroke and systemic
embolism in at risk patients with non-valvular
atrial fibrillation AND in whom:
1. Anticoagulation is inadequate {at least 35%
of the tests are outside of range} following a
reasonable trial {at least 3 months} of
warfarin; OR
2. Anticoagulation with warfarin is
contraindicated or not possible due to
inability to regularly monitor via INR
testing (i.e., No access to INR testing
services at a lab, clinic, pharmacy & home)
Mr. AF
Mr. AF is prescribed warfarin
2 years later, Mr. AF’s wife died and Mr. AF
is unable to cope – started drinking
INR levels fluctuating over 3 months
Time for a new oral anticoagulant
◦ Dabigatran? (Oct 2010, LU April 2012)
◦ Rivaroxaban? (Dec 2012, LU Aug 2013
◦ Apixaban? (Jan 2012, LU July 2012)
LU=Limited Use
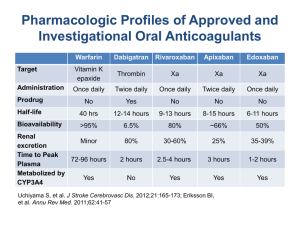
Oral anticoagulants
Direct thrombin inhibitor
Dabigatran
Direct thrombin inhibitor
Half-life: 12-17 hours
Dose: 150mg bid
◦ 110mg bid if ≥ 80y or 75-79y with ≥ 1
bleeding risk factor*
Renal function
◦ CrCl<30mL/min contraindicated
No antidote
No dosette/blisterpack or open capsule
*Bleeding RF = moderate renal impairment (30-50mL/min), P-gp inhibitor, NSAID, anti-platelets,
congenital/aquired coagulation disorders, thrombocytopenia or functional platelet defects,
active/recent ulcerative GI bleeding, recent biopsy or major trauma, recent intracranial hemorrhage,
surgery (brain, spinal or opthalmic), bacterial endocarditis
Dabigatran – Drug Interactions
Contraindicated
◦ Dronedarone, ketoconazole
Avoid: rifampicin
◦ Increase dabigatran concentration:
P-gp inhibitors (i.e., amiodarone, clarithromycin,
cyclosporine, itra-, posa-conazole, quinidine,
tacagrelor, tacrolimus, verapamil, etc)
◦ Decrease dabigatran concentration
P-gp inducers (i.e., carbamazepine, St. John’s Wort,
tenofovir)
Antacids (H2RA, PPI, Al-Mg Hydroxide)
H2RA=Histamine2 Receptor Antagonist, P-gp=P-glycoprotein, PPI=proton pump
inhibitor, Al-Mg=aluminum-magnesium
Dabigatran vs. Warfarin – RE-LY
NEJM 2009;361:1139-51
RCT, dabigatranblinded, warfarinopen-label
Intervention:
◦ Dabigatran 150mg bid vs. dabigatran 110mg bid vs.
warfarinINR 2-3
Inclusion: AF & ≥ 1 of the following:
◦ Previous stroke/TIA, LVEF<40, NYHA class II-IV HF
within 6 months, ≥ 75y or 65-74y + DM, HTN or
CAD
Exclusion:
◦ Severe heart-valve disorder, stroke within 14 days
prior or severe stroke within 6 months prior,
CrCl<30mL/min, active liver disease, conditions
that increase risk of bleed
AF=atrial fibrillation, CAD=coronary artery disease, CrCl=creatinine clearance, DM=diabetes mellitus,
HF=heart failure, HTN=hypertension, LVEF=left ventricular ejection fraction, NYHA=New York Heart
Association, RCT=randomized control trial, TIA=transient ischemic attack, y=year
RE-LY results
NEJM 2009;361:1139-51
N=18,113 non-valvular AF pts at risk of
stroke
CHADS2 mean = 2.1
Mean time in therapeutic range with
warfarin was 64%
Median follow up = 2 years
RE-LY results
Dabigatran (both doses) vs. warfarin
◦
◦
◦
◦
NEJM 2009;361:1139-51
Less hemorrhagic stroke & intracranial bleeds
More dyspepsia
Trend for higher MI?
Higher discontinuation rate with dabigatran
Dabigatran 150mg bid vs. warfarin
◦ Superior to warfarin for stroke/SE (NNT=88)
◦ Superior for ischemic/hemorrhagic stroke
◦ Increase GI bleeds (NNH=100)
Dabigatran 110mg bid vs. warfarin
◦ Non-inferior to warfarin for stroke/SE
◦ Less major bleeds (NNT=77)
Would you give Mr. AF dabigatran?
Yes, maybe?
No, maybe not?
Dabigatran 150mg bid He is on a PPI – potential
superior to warfarin in drug interaction – unclear
stroke or systemic
about clinical significance
embolism
(~14% of RE-LY study
patients were on PPI)
To enhance the absorption of
dabigatran, a low pH is
required – dabigatran
capsules contain dabigatrancoated pellets with a tartaric
acid core
More GI bleed
No antidote
The Hamilton Spectator
February 15, 2014
Trials and errors? Mac, HHS sued over drug
safety
In an unprecedented case, McMaster
University and Hamilton Health Sciences
are facing lawsuits in the United States
over the safety of the drug Pradaxa. As The
Spectator's Steve Buist reports, the lawsuits
allege that regulatory approval for the popular
anticoagulant was partly based on tainted data
from clinical trials led by Hamilton researchers.
http://www.thespec.com/news-story/4369907-trial-and-errors-mac-hhs-suedover-drug-safety/
Oral anticoagulants
Direct thrombin inhibitor
Rivaroxaban
Direct Factor Xa Inhibitor
Half-life: 5-9h (young) or 11-13h (elderly)
Dose: 20mg once daily
◦ CrCl 30-49mL/min: 15mg once daily
Renal function
◦ CrCl < 30mL/min not recommended
No antidote
Rivaroxaban – Drug Interactions
Contraindicated:
Itra- keto- posacon-azoles, ritonavir
CYP 3A4 and P-gp inducers (decrease
rivaroxaban concentration)
◦ Carbamazepine, clarithromycin, phenytoin,
rifampin, St. John’s Wort
Rivaroxaban vs. Warfarin
ROCKET-AF
NEJM 2011;365:883-91
RCT, double-blinded
Intervention:
◦ Rivaroxaban 20mg od vs. warfarinINR 2-3
◦ Rivaroxaban 15mg od if CrCl 30-49mL/min
Inclusion:
◦ Persistent/paroxysmal AF on ≥ 2 episodes, risk of
future stroke/TIA or systemic embolism OR CHADS2
score ≥ 2
Exclusion:
◦ Stroke within 14 days or TIA within 3 days, anemia
Hgb<100g/L, prosthetic heart valve,
CrCl<30mL/min, active liver disease, conditions
that increase risk of bleed
AF=atrial fibrillation, CHADS2=Congestive heart failure, Hypertension, Age≥75, Diabetes, Stroke/Transient
Ischemic Attack, CrCl=creatinine clearance, Hgb=Hemoglobin, RCT=randomized control trial,
TIA=transient ischemic attack, y=year
ROCKET-AF
NEJM 2011;365:883-91
N=14,264 non-valvular AF pts at risk of
stroke
CHADS2 mean = 3.5
Mean time in therapeutic range with
warfarin was 55% (North American sites:
64%)
Median follow up per protocol = 590 days
(1.6 years)
Median follow up intention-to-treat = 707
days (1.9 years)
ROCKET-AF
NEJM 2011;365:883-91
Rivaroxaban vs. warfarin
◦ Rivaroxaban non-inferior to warfarin for stroke
or systemic embolism
◦ Potential Benefits:
Less hemorrhagic stroke (NNT=333) and systemic
embolism (NNT=417)
Less critical bleeding (NNT=167), less fatal bleeding
(NNT=250), less intracranial bleeding (NNT=250)
◦ Potential Harms:
More drop in Hgb ≥ 20g/L (NNH=143), more
transfusions (NNH=200), more GI bleeds (NNH=100),
more epistaxis (NNH=67), more hematuria (NNH=125)
Would you give Mr. AF rivaroxaban?
Yes, maybe?
Rivaroxaban 20mg
once daily noninferior to warfarin
in stroke or systemic
embolism
Once daily dosing
may be more
attractive to Mr. AF
No, maybe not?
CHADS2 score = 1
More GI bleed
No antidote
Oral anticoagulants
Direct thrombin inhibitor
Apixaban
Direct Factor Xa Inhibitor
Half-life: 12 hours
Dose: 5mg twice daily
◦ 2.5mg BID if pts with ≥ 2 of the following:
Age ≥ 80, body weight ≤ 60kg, or Scr ≥ 133 umol/L
Renal function
◦ Excluded patients with CrCl < 25mL/min
◦ CrCl < 15mL/min not recommended
No antidote
Apixaban – Drug Interactions
Contraindications
◦ Itra- keto- posacon-azoles, ritonavir
CYP 3A4 and P-gp inducers (decrease
apixaban concentration)
◦ Carbamazepine, clarithromycin, phenytoin,
rifampin, St. John’s Wort
P-gp inhibitors (increase apixaban
concentration)
◦ Amiodarone, dronedarone, quinidine,
verapamil
Apixaban vs. Warfarin
ARISTOTLE
NEJM 2011;365:981-92
RCT, double-blinded
Intervention:
◦ Apixaban 5mg BID vs. warfarinINR 2-3
◦ Apixaban 2.5mg BID in pts with ≥ 2 of the following:
Age ≥ 80y, body weight ≤ 60kg, or SCr ≥ 133umol/Lmg od
Inclusion:
◦ Permanent/persistent AF or flutter, ≥ 1 of the following
stroke risk factors: age≥75y, prior stroke/TIA/systemic
embolus, HF or LVEF≤40%, DM or HTN
Exclusion:
◦ Stroke within 7 days, Hgb<90g/L, prosthetic heart valve,
renal insufficiency (CrCl<25mL/min or
SCr>221umol/L), active liver disease, conditions that
increase risk of bleed, required ASA > 165mg/d, treatment
with both ASA+thienopyridine
ARISTOTLE Results
NEJM 2011;365:981-92
N=18,201 non-valvular AF pts at risk of
stroke
CHADS2 mean = 2.1
Mean time in therapeutic range with
warfarin was 62.2%
Median follow-up = 1.8 years
ARISTOTLE Results
NEJM 2011;365:981-92
Apixaban vs. Warfarin
◦ Apixaban superior to warfarin for stroke and
systemic embolism (NNT=167/1.8 years)
◦ Potential Benefits:
Decrease stroke (NNT=175), decrease
hemorrhagic stroke (NNT=238) and decrease
mortality (NNT=132)
Decrease major bleed (NNT=67)
Intracranial bleed (NNT=128)
Decreased d/c rates (NNT=45)
Would you give Mr. AF apixaban?
Yes, maybe?
Apixaban 5mg twice
daily superior to
warfarin in stroke or
systemic embolism
Decrease all cause
mortality
No difference in GI
bleeds compared to
warfarin
No, maybe not?
Twice daily?
No antidote
Switching FROM Warfarin NOAC
Check INR
2. Stop warfarin
3. Recheck INR in 2-4 days
Start dabigatran when INR < 2.0CPS
1.
◦ Thrombosis Canada ≤ 2.0
Start rivaroxaban when INR ≤ 2.5CPS
◦ Thrombosis Canada ≤ 2.0
Start apixaban when INR < 2.0CPS
◦ Thrombosis Canada ≤ 2.0
What if?
Mr. AF’s renal function declined:
◦ 72y male, SCr=130umol/L, Ht=65 inches,
Wt=65kg, CrCl=39.5mL/min
What would you give him if he could not
take warfarin?
◦ Dabigatran 150mg or 110mg bid?
◦ Rivaroxaban 20mg or 15mg od?
◦ Apixaban 2.5mg or 5mg bid?
Summary
Warfarin advantages
60+ years experience
Vitamin K antidote
Valvular/non-valvular AF
Allows for missed doses?
No dosage requirements
for renal dysfunction
Monitoring – up to every
3 months
Cost $40/month
Warfarin disadvantages
Many drug/food
interactions
Slow onset
Physician/nurse/pharmaci
st time?
Seasonal changes in INR?
Monitoring?
Summary
Novel oral anticoagulants
Advantages
Less Monitoring:
◦ SCr & CrCl at least
annually
Fast onset
Disadvantages
<2 years experience
No antidote
If miss dose, short
half-life – quick
“offset”
Renal function dose
adjustments
Cost > $100/month
Summary
Warfarin is preferred in:
◦
◦
◦
◦
◦
Mechanical or valvular AF
If INR is stable on warfarin
CrCl < 30mL/min
Liver dysfunction
Poor compliance (or maybe no OAC is
preferred)
◦ Morbidly Obese?
Summary
Dabigatran 150mg bid preferred if recent
ischemic stroke on warfarin
Rivaroxaban or apixaban is preferred:
◦ CrCl 30-50mL/min
◦ Dypepsia or upper GI bleed
◦ Recent acute coronary syndrome
Apixaban preferred if recent GI bleed
Rivaroxaban preferred if poor compliance
with twice daily dosing or request for a
once-daily regimen
Questions?