handout
advertisement

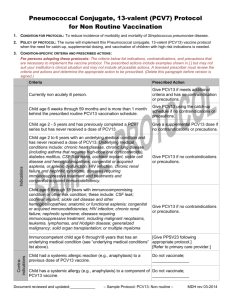
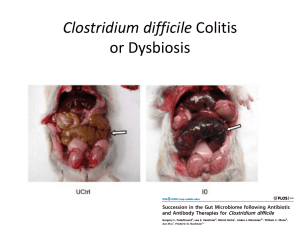
Hot Topics in Infectious Diseases Cont’d Fecal Microbiota Transplantation In treatment for Clostridium Difficile Fecal Microbiota Transplantation • It is the use of indigenous intestinal microorganisms from a healthy donor (via infusion of a liquid suspension of stool) to restore the intestinal microbiota of a diseased individual. Rationale for fecal Transplant • The GIT harbors a highly complex community of microorganisms. • Roles of the microbiota: synthesis of vitamins, carbohydrates fermentaion, bile and hormone and competitive exclusion of pathogens residing in the gut. • Microbiota also influences the development and maturation of the immune system through interactions with the gut epithelium. • Extensive use of antibiotics lead to a selective removal of a group of bacteria species that serve as a barrier to colonization and/or persistence of pathogens. • This causes alterations in the gut barrier functions and result in mucosal immune defects, which would predispose to C diff, allowing spores to germinate and colonize the gut. Fecal microbiota transplantation for recurrent C difficile infection ready for prime time? Agito et al,.Cleve Clin J Med. 2013 Feb;80(2):101-8 Efficacy • Different studies have demonstrated efficacy of fecal microbiota transplantation (FMT) in the treatment of C. difficile-associated diarrhea in patients with recurrent disease after initial antibiotic therapy. • In one review, 92% experienced resolution of symptoms ,89% after a single treatment and 5% after retreatment for failure or relapse. • FMT from related donor showed a slightly higher resolution rate (93%) compared with unrelated donor stool (84%). • Resolutions increased with larger volume of IMT given (97% for 500 cc vs 80% with 200 cc). • Relapses and deaths after FMT were relatively uncommon. • Adverse events: included upper gastrointestinal hemorrhage IBS symptoms, infectious IBS symptoms, constipation have been reported but not attributed to FMT • Stool suspension can be instilled through colonoscopy, enema, or upper GI tract. • Lowest resolution rate was noticed with upper GI route (76%) Indications • Recurrent C difficile infection • Severe C difficile infection not responding to standard therapy after 48 hours. • Mild to moderate C difficile infection with no response to standard treatment for at least 1 week. Fecal microbiota transplantation for recurrent C difficile infection ready for prime time? Agito et al,.Cleve Clin J Med. 2013 Feb;80(2):101-8 DONOR • spouse or significant partner • A family member • Any other healthy donor Fecal microbiota transplantation for recurrent C difficile infection ready for prime time? Agito et al,.Cleve Clin J Med. 2013 Feb;80(2):101-8 Case 3 A 66 y old male patient with hearing loss is being evaluated for cochlear implant. Which of the following vaccines is recommended for him after his implant? 1. 2. 3. 23 valent polysaccharide pneumoccal vaccine. Prevnar 13 conjugate vaccine both • Streptococcus pneumoniae is a leading cause of serious illness, including bacteremia, meningitis, and pneumonia among adults in the United States. • About 4,000 deaths among adults occur each year because of S. pneumoniae. • Incidence of invasive disease is higher in older patients ≥65years or with medical conditions, like hematological diseaes, HIV, functional or anatomic asplenia. POLYSACCHARIDE VACCINE • There are over 90 different capsular types of pneumococci • Pneumovax 23 (PPSV 23) is the available pneumococcal polysaccharide vaccines include 23 purified capsular polysaccharide antigens (serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F). • These serotypes represent 85 to 90 % of the serotypes causing invasive disease in the United States. • PPSV23 is recommended for prevention of invasive disease among all adults aged ≥65 years, and for adults at high risk aged 19–64 years . • Polysaccharide vaccine induces a T-cell-independent immune response, and does not elicit immunologic memory. • Duration of protection is limited to 3 to 5 years. • Reimmunization has been associated with decreased antibody responses, compared with the first dose. • Clinical trials had shown that PPSV23 can protects against invasive pneumococcal disease in adults, yet there is no convincing data about protection against pneumonia, or reduction in all cause mortality. Conjuagate vaccine • In 2010, PCV13 was approved by FDA for use in children and replaced PCV7. • The routine use of PCV7 in infants and young children resulted in significant reductions in invasive pneumococcal disease caused by vaccine serotypes both in children and in adult through herd immunity. • In December 2011, PCV13 was approved for use in adults ≥50 years of age based upon safety and immunogenicity data • In 2012, the ACIP began recommending both PCV13 and PPSV23 for use in individuals aged 19 or older with immunocompromising conditions, cerebrospinal fluid leaks, cochlear implants, chronic renal insufficiency, or nephrotic syndrome. • Conjugate vaccines were developed to produce an enhanced immunological response. • Each polysaccharide antigen is linked chemically to a carrier proteins and so elicit an enhanced immune response . • They resulted in T-cell-dependent memory responses that can be boosted by natural exposure or subsequent vaccination. • The best measurable immune response associated with the efficacy of pneumococcal vaccines is antibody-mediated opsonophagocytic activity (OPA). • In 2 randomized, multicenter immunogenicity studies done in the US and Europe, immunocompetent adults ≥50 years received a single dose of PCV13 or PPSV23 • In adults 60–64 years and >70 years, PCV13 elicited (OPA) antibody responses that were comparable with, or higher than, responses elicited by PPSV23. • OPA antibody response elicited by PCV13 in adults aged 50–59 years for were comparable to OPA response elicited by administration of PCV13 in adults aged 60– 64 years. • Persons who received PPSV23 as the initial study dose had lower OPA antibody responses after subsequent administration of PCV13 than those who received PCV13 as the initial dose . • Data on the immunogenicity of PCV13 in immunocompromised adults are not available. • A randomized, controlled trial of PCV7 efficacy among 496 HIV-infected adults in Malawi demonstrated vaccine efficacy of 75% • Four studies of PCV7 immunogenicity involving 699 HIV-infected patients, were conducted in the US and Europe. Antibody response to a single dose of PCV7 was comparable with PPSV23 for the serotypes evaluated, at all times studied. • When PPSV23 and PCV7 were administered in series, greater immune response was demonstrated when PCV7 was given first . ACIP Recommendations for PCV13 and PPSV23 Use Table • Adults aged ≥19 years with immunocompromising conditions, functional or anatomic asplenia, CSF leaks, or cochlear implants, and who have not previously received any pneumococcal vaccine, should receive a dose of PCV13 first, followed by a dose of PPSV23 at least 8 weeks later. • A second PPSV23 dose is recommended 5 years after the first PPSV23 dose for persons aged 19–64 years with functional or anatomic asplenia and for persons with immunocompromising conditions. • Those who received PPSV23 before age 65 years for any indication should receive another dose of the vaccine at age 65 years, or later if at least 5 years have elapsed since their previous PPSV23 dose • Adults aged ≥19 years with immunocompromising conditions, functional or anatomic asplenia, CSF leaks, or cochlear implants, who previously have received ≥1 doses of PPSV23 should be given a PCV13 dose ≥1 year after the last PPSV23 dose was received. • For those who require additional doses of PPSV23, the first such dose should be given no sooner than 8 weeks after PCV13 and at least 5 years after the most recent dose of PPSV23. Case 4 • A 45 y old female patient presented to ER for significant erythema, swelling and pain of the right forearm. She was diagnosed with skin abscess. She has hx of recurrent SSSI secondary to MRSA. • She underwent I&D and cultures showed MRSA again. MRSA was R to clindamycin, tetracycline but S to bactrim and S to rifampin. She has bactrim allergy in the form of rash. • Which of these is a potential oral option for her? • • • • • • 1. oral vancomycin 2. oral rifmapin 3. Linezolid 4. Tedizolid 5. 3&4 6.ALL of the above. Tedizolid • Tedizolid phosphate (also known asTR-701) is a novel, potent oxazolidinone prodrug rapidly converted in vivo to tedizolid (TR-700),the active form. • Mechanism of action: inhibition of protein synthesis by preventing the formation of the 70S initiation complex, which in turn blocks the initiation of protein synthesis • Tedizolid is active against, MSSA, MRSA, methicillin-susceptible and - resistant coagulase negative staphylococci, vancomycin-susceptible and – resistant enterococci, S. pneumoniae (including resistant strains), S. pyogenes and S. agalactiae, even including linezolid resistant S aureus. • In vitro data shows tedizolid is active against some anaerobic pathogens, mainly Peptostreptococcus spp., Porphyromonas spp., Prevotella spp. and Clostridium perfringens • It can be administered orally or intravenosuly. • 200 mg of oral tedizolid phosphate administered once daily for 5 to 7 days was the lowest effective dose. • available data confirm that tedizolid is 4- to 16-fold more potent than linezolid against staphylococcal and enterococcal isolates • Oral bioavailability was 91.7% • Pulmonary tissue penetration in a murine model of pulmonary infection showed a higher penetration into the epithelial lining fluid than linezolid • It is eliminated primarily through fecal excretion and minor fraction is excreted in the urine. • A phase II multi-center, randomized, double-blind, uncontrolled clinical trial was conducted at eight US sites. • It tested the efficacy of Tedizolid for Rx cSSTI at different doses. • Clinical cure rate was 87.8% in the MITT population and 95.7% in the clinically evaluable population. • Hematological side effects were not observed. The ESTABLISH-1 Randomized Trial • It is a randomized, double-blind, doubledummy, multicenter, multinational, phase 3 noninferiority trial • Examine the efficacy and safety of 200 mg of oral tedizolid phosphate administered once daily for 6 days vs 600 mg of oral linezolid administered twice daily for 10 days for the treatment of adults with ABSSSIs (cellulitis/erysipelas, major cutaneous, abscesses, or wound infections). • Response to treatment was assessed at 48- to 72-hour visit after the first dose of study drug, then at the end-of treatment (EOT; day 11 ), and at the posttherapy evaluation (PTE; 7-14 days) • Response rates at the 48- to 72-hour assessment were 79.5% (95% CI, 74.8% to 83.7%) of 332 patients in the tedizolid phosphate group and 79.4% (95% CI, 74.7% to 83.6%) of 335 patients in the linezolid group. • Sustained clinical treatment response rates at the EOT (day 11) were similar in the tedizolid phosphate and linezolid groups • Treatment response at the PTE visit was also similar for both groups. Conclusion • Treatment of ABSSTIs with 200 mg of tedizolid phosphate once daily for 6 days was statistically noninferior in efficacy to 600 mg of linezolid twice daily for 10 days at both early and late time points. • It was generally well tolerated • Adverse event rates were similar for both groups with fewer GI adverse events among tedizolid phosphate group. • Low platelet counts were less than half as frequent in the tedizolid group as in the linezolid • Preliminary results of preclinical and clinical pharmacology studies suggest that the unique mode of action of tedizolid phosphate, improved pharmacokinetics/pharmacodynamics, lower doses, and lack of monoamine oxidase inhibition in vivo may translate to improved safety vs linezolid. Thank you References • The index case for the fungal meningitis outbreak in the United States. Petit et El, N Engl J Med. 2012 Nov 29;367(22):2119-25. • Multistate fungal meningitis outbreak -- interim guidance for treatment. Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep. 2012 Oct 19;61(41):842. • Multistate outbreak of fungal infection associated with injection of methylprednisolone acetate solution from a single compounding pharmacy - United States, 2012. Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep. 2012 Oct 19;61(41):839-42. • Notice to Clinicians: Continued Vigilance Urged for Fungal Infections among Patients Who Received Contaminated Steroid Injections. Distributed via the CDC Health Alert Network, March 4, 2013, CDCHAN-00342 • Interim Treatment Guidance for Central Nervous System and Parameningeal Infections Associated with Injection of Contaminated Steroid Products. CDC. OCTOBER 23, 2012 • Interim Treatment Guidance for Osteoarticular Infections Associated With Injection of Contaminated Steroid Product. CDC .OCTOBER 23, 2012 References cont’d • Safety and efficacy of fidaxomicin in the treatment of Clostridium difficile-associated diarrhea. Golan et al. Therap Adv Gastroenterol. 2012 Nov;5(6):395-402. • Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Crook et Al. Clin Infect Dis. 2012 Aug;55 Suppl 2:S93-103. • Fidaxomicin (Dificid), a Novel Oral Macrocyclic Antibacterial Agent For the Treatment of Clostridium difficile-Associated Diarrhea in Adults. Cruz MP. P T. 2012 May;37(5):278-8 • Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Cornely et, Clin Infect Dis. 2012 Aug;55 Suppl 2:S154-61. • Clostridium difficile infection: new insights into management. Khanna et al, Mayo Clin Proc. 2012 Nov;87(11):1106-17 References cont’d • Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium difficile Infection Clin Infect Dis. (2011) 53(10): 994-1002 doi:10.1093/cid/cir632. • Fecal microbiota transplantation for recurrent C difficile infection ready for prime time? Agito et al,.Cleve Clin J Med. 2013 Feb;80(2):101-8 • Fecal microbiota transplantation in relapsing Clostridium difficile infection. Rohlke F et al, Therap Adv Gastroenterol. 2012 Nov;5(6):403-20 References Cont’d • Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep. 2012 Oct 12;61(40):816-9. • Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep. 2012 Jun 1;61(21):394-5. • Pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed): a guide to its use in older adults. Scott et al, Drugs Aging. 2012 Oct;29(10):847-55. doi: 10.1007/s40266-012-0017-0 References cont’d • Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. Prokocimer P, De Anda C, Fang E, Mehra P, Das A. JAMA. 2013 Feb 13;309(6):559-69. doi: 10.1001/jama.2013.241 • In vitro activity and microbiological efficacy of tedizolid (TR-700) against Gram-positive clinical isolates from a phase 2 study of oral tedizolid phosphate (TR-701) in patients with complicated skin and skin structure infections. Prokocimer P, Bien P, Deanda C, Pillar CM, Bartizal K. Antimicrob Agents Chemother. 2012 Sep;56(9):4608-13. doi: 10.1128/AAC.00458-12. Epub 2012 Jun 11 • Tedizolid (TR-701): a new oxazolidinone with enhanced potency. Kanafani ZA, Corey GR. Expert Opin Investig Drugs. 2012 Apr;21(4):515-22. doi: 10.1517/13543784.2012.660250. Epub 2012 Feb 13. Review