(PCV13) Protocol for Non Routine Vaccination
advertisement

Pneumococcal Conjugate, 13-valent (PCV7) Protocol
for Non Routine Vaccination
1. CONDITION FOR PROTOCOL: To reduce incidence of morbidity and mortality of Streptococcus pneumoniae disease.
2. POLICY OF PROTOCOL: The nurse will implement this Pneumococcal conjugate, 13-valent (PCV13) vaccine protocol
when the need for catch-up, supplemental dosing, and vaccination of children with high risk indications is needed.
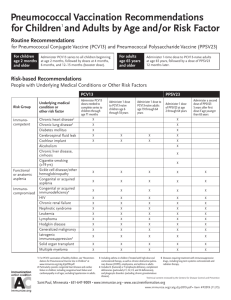
Indications
3. CONDITION-SPECIFIC CRITERIA AND PRESCRIBED ACTIONS:
For persons adopting these protocols: The criteria below list indications, contraindications, and precautions that
are necessary to implement the vaccine protocol. The prescribed actions include examples shown in [ ] but may not
suit your institution’s clinical situation and may not include all possible actions. A licensed prescriber must review the
criteria and actions and determine the appropriate action to be prescribed. (Delete this paragraph before version is
signed.)
Criteria
Prescribed Action
Currently non acutely ill person.
Give PCV13 if meets additional
criteria and has no contraindication
or precautions.
Child age 6 weeks through 59 months and is more than 1 month
behind the prescribed routine PCV13 vaccination schedule.
Give PCV13 using the catch-up
schedule if no contraindications or
precautions.
Child age 2 - 5 years and has previously completed a PCV7
series but has never received a dose of PCV13.
Give a supplemental PCV13 dose if
no contraindications or precautions.
Child age 2 to 6 years with an underlying medical condition and
has never received a dose of PCV13. Underlying medical
conditions include: chronic heart disease, chronic lung disease
(including asthma that requires high-dose oral corticosteroids),
diabetes mellitus, CSF fluid leaks, cochlear implant, sickle cell
disease and hemoglobinopathies, congenital or acquired
asplenia, or splenic dysfunction, HIV infection, chronic renal
failure and nephritic syndrome, diseases requiring
immunosuppressive treatment and treatments and
congenital/acquired immunodeficiency.
Give PCV13 if no contraindications
or precautions.
Child age 6 through 18 years with immunocompromising
condition or other risk condition; these include: CSF leak;
cochlear implant; sickle cell disease and other
hemoglobinopathies; anatomic or functional asplenia; congenital
or acquired immunodeficiencies; HIV infection; chronic renal
failure; nephrotic syndrome; diseases requiring
immunosuppressive treatment, including malignant neoplasms,
leukemia, lymphomas, and Hodgkin disease; generalized
malignancy; solid organ transplantation; or multiple myeloma
Give PCV13 if no contraindications
or precautions.
Contraindications
Immunocompetent child age 6 through18 years that has an
[Give PPSV23 following
underlying medical condition (see “underlying medical conditions” appropriate protocol.]
list above).
[Refer to primary care provider.]
Child had a systemic allergic reaction (e.g., anaphylaxis) to a
previous dose of PCV13 vaccine.
Do not vaccinate;
________________
Child has a systemic allergy (e.g., anaphylaxis) to a component of Do not vaccinate;
PCV13 vaccine.
________________
Document reviewed and updated:____________
– Sample Protocol: PCV13: Non routine –
MDH rev 03-2014
Precautions
Pneumococcal Conjugate, 13-valent (PCV7) Protocol
for Non Routine Vaccination
Child is currently on antibiotic therapy.
Proceed to vaccinate.
Child has a mild illness defined as temperature less than
____°F/°C with symptoms such as: {to be determined by medical
prescriber}
Proceed to vaccinate.
Child has a moderate to severe illness defined as temperature of
Defer vaccination and {to be
____°F/°C or higher with symptoms such as: {to be determined by
determined by medical prescriber}
medical prescriber}
4. PRESCRIPTION:
Supplemental dose: Give one dose of PCV13 0.5 ml, IM.
Child age 2 to 6 years with underlying medical condition including immunocompromising conditions without
previous PCV13 dose: Give two doses 8 weeks apart, 0.5 ml, IM.
These children should receive PPSV23 8 or more weeks following PCV13 vaccination – see PPSV23 protocol.
Child 6 through 18 years with immunocompromising condition without previous PCV13 dose:
Give PCV13 0.5 ml, IM.
These children should receive PPSV23 8 or more weeks following PCV13 vaccination if they have not previously
received PPSV23 – see PPSV23 protocol.
Catch-up vaccination: Give PCV13 0.5 ml, IM, using the following catch-up guidance found in this table:
Minimum interval between doses
Dose 1 to dose 2
Dose 2 to dose 3
Dose 3 to dose 4
4 weeks if first dose given younger
than age 12 months
8 weeks (as final dose for healthy
children), if first dose given at age 12
months or older or current age is
24-59 months
No further doses needed for healthy
children if first dose given at age 24
months or older.
4 weeks if current age under 12
months
8 weeks (as final dose for healthy
children), if current age 12 months
or older
No further doses needed for
healthy children if previous dose
given at age 24 months or older
8 weeks (as final dose)
The 4th dose is only necessary for
children age 12 months to 5 years
who received 3 doses before age
12 months or for high-risk children
who received 3 doses at any age.
For children aged 24 through 59 months with underlying medical conditions, give 1 dose of PCV if 3 doses were
received previously; or give 2 doses of PCV at least 8 weeks apart if fewer than 3 doses were received previously.
Document reviewed and updated:____________
– Sample Protocol: PCV13: Non routine –
MDH rev 03-2014
5. EMERGENCY OR ANAPHYLAXIS: [Depending on clinic staffing, include one of the two options below.]
In the event of a medical emergency related to the administration of a vaccine. RN will apply protocols as described in
____________________________________________________________________________________________.
In the event of an onset of symptoms of anaphylaxis including:
o rash
o itchiness of throat
o difficulty breathing
o bodily collapse
o swollen tongue or throat
LPN or unlicensed assistive personnel (MA) will immediately contact the RN in order to implement the
____________________________________________________________________________________________.
6. QUESTIONS OR CONCERNS:
In the event of questions or concerns, call Dr. ____________________________at _____________________________.
This protocol shall remain in effect for all patients of ______________________________until rescinded or until
_____________________________________.
Name of prescriber: _______________________________________________________________________________
Signature: ________________________________________________________________________________________
Date: ___________________________
Document reviewed and updated:____________
– Sample Protocol: PCV13: Non routine –
MDH rev 03-2014