Chapter 19: Electric Charges and Currents
advertisement

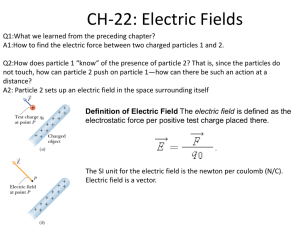
Chapter 19: Electric Charges and Currents 19-1: Electric Charge 19-2: Static Electricity Electric Charge • All matter is made up of atoms, and atoms are made up of tiny particles called protons, neutrons, and electrons. • Both protons and electrons have a basic property called charge - protons are positive and electrons are negative. Charge and Force • A force of attraction pulls objects together - this exists between 2 oppositely charged particles (negative-positive) • A force of repulsion pushes objects apart - this exists between 2 like charges (neg-neg, pos-pos) Rule for Charges: Like charges repel each other, unlike charges attract each other Electric Field • Definition: the arrangement of field lines that determine the force per unit charge a small stationary charge would experience at each point in space • The pattern of an electric field depends on the number, size, sign, and location of charges. • The electric field is visualized by showing lines of force • Force is stronger where lines are closer together occurs when charges are close • The direction of the force depends on whether the placed charge is positive or negative Electric Field - Like Charges Electric Field - Unlike Charges Electric Field - Charges 19-2: Static Electricity • Electrons may be loosely held to their atoms so they can easily be separated from their atoms. • An entire object can acquire a charge • A neutral object acquires an electric charge when it either gains or loses electrons. Methods of Gaining or Losing Charges: • Friction: removes the charges from an object and places them on another object. Methods of Gaining or Losing Charges: • Conduction: electrons flow through one object to another by direct contact. Methods of Gaining or Losing Charges: • Induction: charges are rearranged and line up in different positions. Conductors and Insulators • Conductors: material which permits electrons to flow freely or transfers heat more easily than other substances. Ex: metals – silver, copper, aluminum • Insulators: material made up of atoms with tightly bound electrons that are unable to flow freely – do not conduct electric charges well. Ex: rubber, glass, wood, plastic, dry air • Polarized: partial alignment of negative and positive charges in molecules in response to an electric field Electric Discharge • Transfer of electrons from one object to another results in a static charge on each object. • Static electricity is the build up of unbalanced electric charges on an object • Electrons that move from one object to another eventually leave the object • Electric discharge: loss of static electricity as electric charges move off an object Lightning • Charges separate within the thunderclouds • There is a build up positive and negative charges in different parts of the cloud • Objects on Earth become electrically charged by induction • Charges move away from the cloud, positive charges are left closest to ground – transfer of electrons is lightning • Lightning can be cloud to cloud • Thunder is the expansion of air from the heat produced by lightning Lightning Rods and Grounding • Benjamin Franklin – lightning rods • Grounding: the Earth is extremely large and is a good conductor of electric charge. Earth can easily accept or give up electric charges. • Lightning rod is put on top of buildings. A wire is run from the rod to the ground where lightning can pass through safely. The charge then goes into Earth. Lightning Rods and Grounding Electroscope • Can detect electric charges • Consists of 2 leaves, a metal knob, and a metal rod • A charge is put onto the knob which then flows down the metal rod into the leaves separating them. • If an electroscope is touched by a negative charge, the negative charge flows down the rod into the leaves, giving both leaves a negative charge and causing them to separate (like charges repel). Same thing will happen if a positive charge is put to the electroscope.