File
advertisement
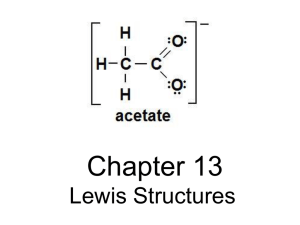
Lewis Structures For Covalent Compounds NAB Method Information… • Octet rule alone does not allow us to create correct Lewis Structures • Does not tell us where or how to place the bonded electrons • NAB method helps N = “NEEDED” 1. Calculate the “N” as the sum of the electrons necessary for all elements to achieve an octet – Important exceptions (as usual) • • • H=2 Be = 4 B=6 NEEDED Add together the number of valence electrons for each atom in the molecule if each had a full octet. For example, CF4 There are 5 atoms in the molecule. If each had a full octet, 5 x 8 = 40 e-s are needed A “AVAILABLE” 2. Calculate “A” as the sum of all valence electrons available to share (total number of valence electrons for ALL atoms involved) AVAILABLE ELECTRONS Add together the number of valence electrons for each atom in the molecule. For example, CF4 Carbon has four valence electrons and each fluorine has seven valence electrons = 4 + 4(7) = 32 available e- “B” BONDS 3. This is easy – Shared is the difference between NEEDED and AVAILABLE: N - A. – Each bond requires 2 electrons – So B = (N – A) / 2 - B is the number of bonds that will come off the central atom BONDS For example, CF4 The number of shared electrons is N – A = 40 – 32 = 8 shared electrons 2 electrons per bond, so 8/2 = 4 bonds Steps for Creating Lewis Structure of Molecules 1. Create a “reasonable” skeleton structure 2. The least electronegative element will be the central atom 3. Never H or any of the Halogens • WHY??? Write out the elements of the molecule so that the least electronegative elements is in the center surrounded by the other elements. For example, CF4 F F C F F How do you choose the central atom? Many times it's readily apparent: SiBr4 CH4 H2S CO2 Place a covalent bond between the central atom and the outside atoms. Remember each covalent bond contains two electrons. F F C F F The four covalent bonds use eight of the 32 valence electrons in CF4 F F C F F • This uses 24 electrons. There are no electrons left, so this is The Lewis dot structure for CF4 There are 24 valence electrons remaining. Add electrons to the outer atoms as lone pairs to satisfy the Octet Rule. • Rule 1: NAB • Rule 2: Place the least electronegative element at the center, except for H which is always an outer atom • Rule 3: Add covalent bonds between the center atom and the outer atoms • Rule 4: Add lone pairs to the outer atoms • Rule 5: Add lone pairs to the center atom Number of Bonds H: halogens and hydrogen can only have 1 bond O: oxygen group can have 2 bonds N: nitrogen group can have 3 bonds C: carbon group can have 4 bonds Be and B, Al: too small to hold a full octet; Be forms only 2 bonds and B and Al form only 3 bonds RESONANCE Consider the Lewis structure of the carbonate ion, CO32-. The Lewis structure for this ion has a carbon-oxygen double bond, and two carbon-oxygen single bonds. But which of the three oxygens forms the double bond? There are three possibilities: When more than one Lewis structure can be drawn, the molecule or ion is said to have resonance We use a double headed arrow to show that individual structures are related by resonance. HYPERVALENCY Larger atoms can exhibit hypervalency, in which the octet is expanded and there are more than eight electrons in the valence orbitals. Valence expansion (also known as hypervalency) is allowed for atoms in the 3rd period and below of the periodic table. Hypervalency