8.4 notes
advertisement

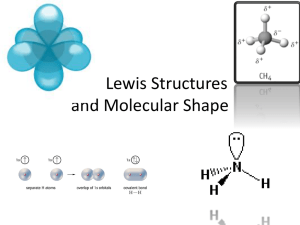
Section 8.4 Molecular Shapes • Summarize the VSEPR bonding theory. • Predict the shape of, and the bond angles in, a molecule. • Define hybridization. atomic orbital: the region around an atom’s nucleus that defines an electron’s probable location VSEPR model hybridization The VSEPR model is used to determine molecular shape. VSEPR Model • The shape of a molecule determines many of its physical and chemical properties. • Molecular geometry (shape) can be determined with the Valence Shell Electron Pair Repulsion model, or VSEPR model which minimizes the repulsion of shared and unshared electrons around the central atom. VSEPR Model (cont.) • Electron pairs repel each other and cause molecules to be in fixed positions relative to each other. • Unshared electron pairs also determine the shape of a molecule. • Electron pairs are located in a molecule as far apart as they can be. VSEPR Theory Types of e- Pairs (around central atom) Bonding Areas - from bonds (single, double, triple) all count as one pair Lone pairs - nonbonding e- Lone pairs repel more strongly than bonding pairs!!! Determining Molecular Shape Draw the Lewis Diagram. Tally up e- pairs on central atom. Single/double/triple bonds = ONE pair (AREA) Shape is determined by the # of bonding AREAS and LONE PAIRS. Know the 8 common shapes & their bond angles! Common Molecular Shapes e- Tally 2 total 2 bond areas 0 lone pairs LINEAR BeH2 180° Common Molecular Shapes e- Tally 3 total 3 bond areas 0 lone pairs BF3 TRIGONAL PLANAR 120° Common Molecular Shapes e- Tally 4 total 4 bond areas 0 lone pairs CH4 TETRAHEDRAL 109.5° Common Molecular Shapes e- Tally 4 total 3 bond areas 1 lone pair NH3 TRIGONAL PYRAMIDAL 107° Common Molecular Shapes e- Tally 3 total 2 bond areas 1 lone pair SO2 BENT <120° Common Molecular Shapes e- Tally 4 total 2 bond areas 2 lone pairs H2O BENT 104.5° Common Molecular Shapes e- Tally 5 total 5 bond areas 0 lone pairs PCl5 TRIGONAL BIPYRAMIDAL 120°/90° Common Molecular Shapes e- Tally 6 total 6 bond areas 0 lone pairs SF6 OCTAHEDRAL 90° Examples PF3 4 total 3 bond areas 1 lone pair F P F F TRIGONAL PYRAMIDAL 107° Examples CO2 2 total 2 bond areas 0 lone pair O C O LINEAR 180° Hybridization Is the process by which s, p, and d orbitals combine to form new identical orbitals The hybridization is equal to the total number of Bonding Areas and lone pairs around the central atom in the molecule. For example: water has two bonding areas and two lone pairs of electrons, so we need 4 total hybrid orbitals = one s orbital and three p orbitals = sp3 Hybridization (cont.) • Single, double, and triple bonds occupy only one hybrid orbital (CO2 with two double bonds forms an sp hybrid orbital). Hybridization (cont.) Hybridization (cont.) Hybridization (cont.) Section 8.4 Assessment The two lone pairs of electrons on a water molecule do what to the bond angle between the hydrogen atoms and the oxygen atom? A. They attract the hydrogen atoms and increase the angle greater than 109.5°. 0% A B C D 0% 0% D 0% A D. They create resonance structures with more than one correct angle. C C. They do no affect the bond angle. A. B. C. D. B B. They occupy more space and squeeze the hydrogen atoms closer together. Section 8.4 Assessment The sp3 hybrid orbital in CH4 has what shape? A. linear B. trigonal planar D A 0% C D. octahedral A. A B. B C. C 0% 0% 0% D. D B C. tetrahedral