Chapter 9 Covalent Bonding: Orbitals
advertisement

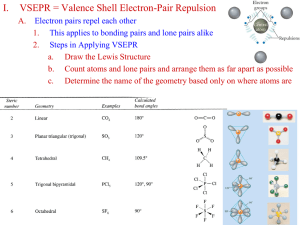
Chapter 9 Covalent Bonding: Orbitals Hybridization • The mixing of atomic orbitals to form special orbitals for bonding. • The atoms are responding as needed to give the minimum energy for the molecule. The Valence Orbitals on a Free Carbon Atom: 2s, 2px, 2py, and 2pz The Formation of sp3 Hybrid Orbitals An Energy-Level Diagram Showing the Formation of Four sp3 Orbitals Tetrahedral Set of Four sp3 Orbitals The Nitrogen Atom in Ammonia is sp3 Hybridized sp3 Hybridization The experimentally known structure of CH4 molecule can be explained if we assume that the carbon atom adopts a special set of atomic orbitals. These new orbital are obtained by combining the 2s and the three 2p orbitals of the carbon atom to produce four identically shaped orbital that are oriented toward the corners of a tetrahedron and are used to bond to the hydrogen atoms. Whenever a set of equivalent tetrahedral atomic orbitals is required by an atom, this model assumes that the atom adopts a set of sp3 orbitals; the atom becomes sp3 hybridized. The Hybridization of the s, px, and py Atomic Orbitals An Orbital Energy-Level Diagram for sp2 Hybridization • A sigma () bond centers along the internuclear axis. • A pi () bond occupies the space above and below the internuclear axis. H H C C H H An sp2 Hybridized C Atom The Bonds in Ethylene Sigma and Pi Bonding The Orbitals for C2H4 When One s Orbital and One p Orbital are Hybridized, a Set of Two sp Orbitals Oriented at 180 Degrees Results The Hybrid Orbitals in the CO2 Molecule The Orbital Energy-Level Diagram for the Formation of sp Hybrid Orbitals on Carbon The Orbitals of an sp Hybridized Carbon Atom The Orbital Arrangement for an sp2 Hybridized Oxygen Atom The Orbitals for CO2 The Orbitals for N2 A Set of dsp3 Hybrid Orbitals on a Phosphorus Atom An Octahedral Set of d2sp3 Orbitals on a Sulfur Atom The Relationship of the Number of Effective Pairs, Their Spatial Arrangement, and the Hybrid Orbital Set Required The Localized Electron Model Three Steps: Draw the Lewis structure(s) Determine the arrangement of electron pairs (VSEPR model). Specify the necessary hybrid orbitals. Molecular Orbitals (MO) • Analagous to atomic orbitals for atoms, MOs are the quantum mechanical solutions to the organization of valence electrons in molecules. • Molecular orbitals have many of the same characteristics as atomic orbitals, such as they can hold two electrons with opposite spins and the square of the molecular orbital wave function indicates electron probability. The Combination of Hydrogen 1s Atomic Orbitals to Form Molecular Orbitals The Molecular Orbitals for H2 Types of MOs • bonding: lower in energy than the atomic orbitals from which it is composed. Electrons in this type of orbital will favor the molecule. • antibonding: higher in energy than the atomic orbitals from which it is composed. Electrons in this type of orbital will favor the separated atoms. Bonding and Antibonding Molecular Orbitals (MOs) The Molecular Orbital Energy-Level Diagram for the H2 Molecule The Molecular Orbital Energy-Level Diagram for the H2- Ion Bond Order (BO) • Difference between the number of bonding electrons and number of antibonding electrons divided by two. # bonding electrons # antibonding electons BO = 2 • Bonds order is an indication of bond strength. Large bond order means greater bond strength. The Molecular Orbital Energy-Level Diagram for the He2 Molecule Bonding in Homonuclear Diatomic Molecules In order to participate in MOs, atomic orbitals must overlap in space. (Therefore, only valence orbitals of atoms contribute significantly to MOs.) The Relative Sizes of the Lithium 1s and 2s Atomic Orbitals The Molecular Orbital Energy-Level Diagram for the Li2 Molecule The Molecular Orbitals from p Atomic Orbitals The Expected Molecular Orbital Energy-Level Diagram Resulting from the Combination of the 2p Orbitals on Two Boron Atoms The Expected Molecular Orbital Energy-Level Diagram for the B2 Molecule Paramagnetism unpaired electrons attracted to induced magnetic field much stronger than diamagnetism Diamagnetism paired electrons repelled from induced magnetic field much weaker than paramagnetism Diagram of the Kind of Apparatus Used to Measure the Paramagnetism of a Sample The Correct Molecular Orbital Energy-Level Diagram for the B2 Molecule Molecular Orbital Summary of Second Row Diatomics Outcomes of MO Model 1. As bond order increases, bond energy increases and bond length decreases. 2. Bond order is not absolutely associated with a particular bond energy. 3. N2 has a triple bond, and a correspondingly high bond energy. 4. O2 is paramagnetic. This is predicted by the MO model, not by the LE model, which predicts diamagnetism. Combining LE and MO Models bonds can be described as being localized. bonding must be treated as being delocalized. The Resonance Structures for O3 and NO3- A Benzene Ring The Sigma System for Benzene The Pi System for Benzene The NO3- Ion